1488
Imaging cerebral ketone metabolism with hyperpolarized 13C magnetic resonance spectroscopy1Physical Therapy and Rehabilitation Science, University of California, San Francisco, San Francisco, CA, United States, 2Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 3Bioengineering, University of California, Berkeley, Berkeley, CA, United States
Synopsis
As ketogenic diets have been shown to elicit cognitive improvements in Alzheimer’s disease (AD) patients, non-invasive methods allowing for in vivo monitoring of brain ketone metabolism are critically needed to understand and monitor these observations. We hypothesized that 13C magnetic resonance spectroscopy of hyperpolarized (HP) [1-13C] beta-hydroxybutyrate (BHB) could be used to monitor response to ketogenic diets in health and AD. Here, we characterized HP [1-13C] BHB and validated an AD mouse model for application of our HP probe. We also carried out the first proof-of-concept acquisition of data showing in vivo metabolism of HP BHB in the mouse brain.
Introduction
Ketogenic diets (KDs) are increasingly being investigated as treatment for mild or moderate Alzheimer’s disease (AD) patients, where some cognitive improvements were observed post-diet1–5. Most commonly, monitoring ketone administration in humans involves measuring the ketone in blood serum whose concentration is highest - beta-hydroxybutyrate (BHB)6. However, in the presence of increased serum BHB, studies have shown both increased and decreased cerebral activity of the enzyme beta-hydroxybutyrate dehydrogenase (BDH), which converts BHB to Acetoacetate (AcAc)7. Increased blood ketone level is thus not always coupled to increased BDH activity, and an in vivo measurement of cerebral BHB metabolism is critically needed. We hypothesized that 13C magnetic resonance spectroscopy (MRS) of hyperpolarized (HP) [1-13C] BHB could provide such an in vivo assessment of ketone metabolism. In this work, we measured relaxation times (T1) and polarization level of HP [1-13C] BHB, confirmed that enzymatic conversion to AcAc by BDH could be detected, and characterized the mouse model of AD in which we will apply our HP probe. Finally, we carried out the first in vivo acquisition of cerebral metabolic data following injection of HP [1-13C] BHB.Methods
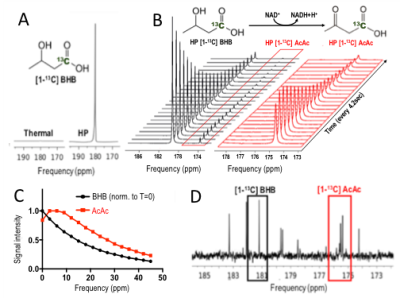
HP Probe: [1-13C] BHB preparation (100ul for enzyme experiments, 30ul otherwise, of 6.4M in ddH2O) was polarized for 1 hour (Hypersense polarizer, Oxford Instruments). Dissolution in buffer (Tris-HCl and EDTA in dH2O) produced a solution of 130mM/40mM BHB at pH 7 and 37°C. Solution T1 was measured at 1.5T (benchtop Pulsar scanner), 3T (Bruker BioSpec) and 11.7T (Agilent vertical bore).Enzyme experiments: 2.5ml HP [1-13C] BHB was rapidly added to an NMR tube containing 100U BDH and 31mg NAD, then placed into the 11.7T MR system. Data were acquired every 4.2 seconds for 5 minutes.
In vivo 13C labeling: 300ul of 13C BHB (40mM) was injected into an anesthetized C57BL/6J mouse via the tail vein. The mouse was euthanized within 1 minute of injection to mimic the quick HP acquisitions, and the brain snap frozen. Tissue metabolites were extracted using chloroform/methanol, and 13C data acquired on a 500MHz Bruker spectrometer (1D decoupled zgpr sequence with f1 presaturation, FA=30°)
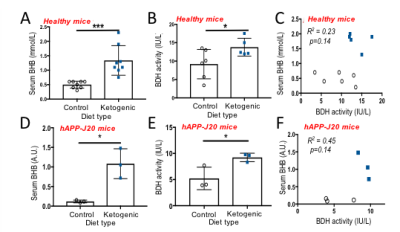
AD model validation: C57BL/6J or the AD model hAPP-J20 mice (n=6-8, n=3 respectively) were placed on either a KD (TD.160153, Envigo) or control diet (TD.150345, Envigo). Serum BHB levels were assessed by tail prick (Precision Xtra strips). Brain BDH activity was assessed following kit instructions (Biomedical Research Service, University of Buffalo).
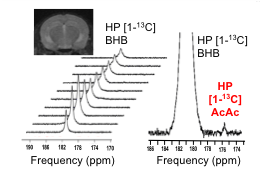
In vivo proof of concept: One anesthetized C57BL/6J mouse (fasted) was placed into the 3T MR system with a dual tuned 1H/13C surface head coil (built in-house) over the brain. 300ul HP [1-13C] BHB was injected via a tail vein catheter. Dynamic, non-localized data were acquired following injection (TR=3.2s; 30 repetitions; FA=15°; RG=203).
Data analysis and statistics: T1 and enzyme data were analyzed using Mestrenova. In vivo data were analyzed in jMRUI. Student’s t-tests were used to compare between-diet serum BHB levels and BDH activity, and a Pearson correlation used to test correlation between parameters.
Results
T1 was 41s at 1.5T ; 37s at 3T; 28s at 11.7T, and polarization level was 21% (Figure 1A), comparable to other HP probes8. Metabolism of HP [1-13C] BHB (181ppm) to HP 13C AcAc (175ppm) by BDH could be detected (Figure 1B-C). Both 13C BHB and 13C AcAc resonances were observed in brain tissue extracts, demonstrating cerebral metabolism of the injected probe during the timeframe of the HP experiment (Figure 1D). Serum BHB was significantly elevated (168%, p<0.005) in healthy mice fed a KD compared to control diet (Figure 2A); BDH activity was also significantly elevated (50%, p=0.05, Figure 2B). However, importantly, there was no correlation between serum BHB and brain BDH activity (Figure 2C). In the AD mouse model (hAPP-J20), serum BHB was also significantly elevated (>800%, p=0.01) in KD-fed mice compared to control-diet-fed (Figure 2D), along with brain BDH activity (77%, p=0.04, Figure 2E). As for healthy mice, there was no correlation between serum BHB and brain BDH activity (Figure 2F). These data fully justify the need for an in vivo measure of brain ketone metabolism. Following intravenous injection, HP [1-13C] BHB was observed in the brain at 3T over multiple timepoints (Figure 3), and on summing the data, HP 13C AcAc was detected. To our knowledge, this is the first observation of HP [1-13C] BHB metabolism in the mouse brain.Discussion and conclusions
Enzymatic conversion of HP [1-13C] BHB to AcAc by BDH occurs within the lifetime of our HP probe, both with isolated enzyme and in the in vivo mouse brain, highlighting the potential of this probe. While KD increased BHB serum levels and cerebral BDH activity in both healthy and AD mice, our results demonstrate that these two values are not correlated, confirming that serum BHB is an unsuitable marker of ketone metabolism following a KD. This further justifies the need for an in vivo method for assessing BDH activity, which would provide valuable information on the in vivo metabolic status of the AD brain. Finally, we observed HP [1-13C] BHB to AcAc conversion in vivo at 3T, demonstrating the feasibility of our approach. Future imaging studies will assess control and KD-fed hAPP-J20 mice, and compare to healthy animals.Acknowledgements
This work was supported by research grants: NIH R01NS102156, NIH RF1AG064170, Cal-BRAIN 349087, Hilton Foundation – Marilyn Hilton Award for Innovation in MS Research #17319. Dana Foundation: The David Mahoney Neuroimaging program, NIH Hyperpolarized MRI Technology Resource Center #P41EB013598, and a seed grant from the UCSF Department of Radiology and Biomedical Imaging (#19-04).References
1. Kashiwaya Y, Bergman C, Lee J-H, et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34(6):1530-1539.
2. Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement Transl Res Clin Interv. 2018;4:28-36.
3. Croteau E, Castellano C-A, Richard MA, et al. Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer’s Disease. Swerdlow R, ed. J Alzheimer’s Dis. 2018;64(2):551-561.
4. Reger MA, Henderson ST, Hale C, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25(3):311-314.
5. Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond). 2009;6(1):31.
6. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412-426.
7. Grabacka M, Pierzchalska M, Dean M, Reiss K. Regulation of Ketone Body Metabolism and the Role of PPARα. Int J Mol Sci. 2016;17(12):2093.
8. Najac C, Radoul M, Le Page LM, et al. In vivo investigation of hyperpolarized [1,3-13C2]acetoacetate as a metabolic probe in normal brain and in glioma. Sci Rep. 2019;9(1):3402.
Figures