1485
FUNCTIONAL DISCONNECT PRECEDES STRUCTURAL ATROPHY IN CONFIRMED MULTI-DOMAIN AMNESTIC MILD COGNITIVE IMPAIRMENT1University of the Sunshine Coast, Sunshine Coast, Australia
Synopsis
Recent work suggests that aberrant functional neurocircuitries arise prior to significant structural atrophy and clinically cognitive deficits in dementia. Using a rigorous, longitudinally confirmed multi-domain amnestic MCI cohort we show that functional decoupling of networks implicated in memory and executive function, occur prior to measurable MTL atrophy in this population. Moreover, we show that decreased functional connectivity in these networks is associated with poorer cognitive performance. Further longitudinal studies investigating the neuronal underpinnings of disease progression will provide insight into potential functional biomarkers and establish significance of functional decoupling within the sequential model of dynamic biomarkers of Alzheimer’s pathological cascade.
Background
Clinical diagnosis of dementia is preceded by an extended preclinical period of neurodegeneration without clinical symptoms, suggesting that a cascade of dynamic biomarkers precede clinical onset 1. This has led to a field-wide focused attempt to identify potential biomarkers of preclinical AD (predominantly focused around amyloidosis) and to the conceptualisation and operationalisation of Mild Cognitive. Currently, MCI is considered to be the earliest phase of cognitive decline preceding clinical diagnosis of dementia that can be detected by conventional clinical assessments and is therefore an important diagnostic entity in both clinical and research settings. However, the on ongoing problem in MCI research is the high rate of false positive MCI diagnosis when a single assessment point is used, with multiple longitudinal studies reporting that 28-56% of those diagnosed with MCI are found to recover to unimpaired levels of functioning 2-6. The use of unrefined mild cognitive impairment (MCI) diagnostic criteria in many studies has hampered the investigation of biomarker relationships. multi-domain amnestic MCI (mdaMCI) has the highest reliability of diagnosis and temporal stability. Furthermore, there is emerging evidence suggesting that clinically detected deterioration of executive functions are seen in MCI and may precede episodic memory deficits in early stages of AD 6 . Functional networks within the limbic system, frontal and temporal cortex have been implicated in higher order executive functioning, impulse control, cognitive flexibility and decision making 7-8. It has been suggested that the functional disruptions within these neurocircuitries lead to maladaptive responses during cognitive and emotional processing, which may underpin cognitive decline in this cohort 9. Here we present structural and rsfMRI findings from a pilot study carried out in older adults with longitudinally confirmed mdaMCI or AD compared with health comparators. With this preliminary study we aim 1: to investigate the cortical and subcortical atrophy patterns and corresponding resting-state functional network signatures that distinguish HC, mdaMCI and mild AD (mAD). 2: to examine the association between neural network dysfunction and memory and executive deficits in mdaMCI and AD.Methods
Neuro MRI scans were acquired in 44 participants (HC = 24, mdaMCI = 14 and AD = 6) on a 3-Tesla Siemens Skyra MRI (Germany, Erlangen) with a 64-channel head and neck receive coil recruited at the Sunshine Coast Mind and Neuroscience Thompson Institute (SCMN-TI). High-resolution, whole-brain, anatomical scans were acquired using a MPRAGE; TR=2200ms, TE=1.76ms, TI=850ms, FOV=240mm, 256x256 matrix, spatial resolution=0.9mm isotropic. rsfMRI connectivity were analysed from multi-slice, T2* echo-planar BOLD sequences acquired with eyes closed (FOV=240x240mm, matrix size=80x80, 56 slices, slice thickness=3mm, TR/TE=1400/30ms, 404 volumes, multi-slice factor=4, acceleration factor=2, scan duration=9 minutes). Cortical reconstruction and volumetric segmentations using the whole-brain imaging analysis stream in FreeSurfer v6.0. Significant differences in left and right whole, head and body hippocampal volume segmentations were assessed with a One-Way ANCOVA. Post-hoc Bonferroni multiple comparisons analysis was used to determine group level significance. Vertex-wise surfaced-based group analysis to determine significant difference in cortical thickness between groups were carried out in FreeSurfer’s general linear model analysis suite 10. Group differences in functional connectivity were investigated using the Matlab-based CONN toolbox 11. A One-Way ANCOVA analysis was carried out to investigate differences in (i) whole brain ROI-to-ROI functional connectivity (excluding brain stem and cerebellar), and (ii) a focused analysis of functional connectivity within 56 fronto-temporal-limbic ROIs between HC and mdaMCI and mdaMCI and mAD. Finally, a correlation analysis was constructed to investigate the association between six memory and executive domain performance scores and fronto-temporal-limbic functional connectivity across the entire participant sample.Results
Hippocampal subvolumes differentiated mAD from HC and mdaMCI. However, hippocampal segmentation volumes did not differentiate between HC and mdaMCI groups. Uncorrected vertex-wise surface analysis revealed very little difference in whole brain cortical thickness between HC and mdaMCI (Figure 1). A significant pattern of cortical thinning in the frontal and temporal lobes was observed when comparing HC versus mAD and mdaMCI versus mAD. However, this did not survive multiple comparison thresholding. Functional decoupling of fronto-temporal networks implicated in memory and executive function differentiated HC and mdaMCI (Figure 2). Decreased functional connectivity in these networks was associated with poorer cognitive performance scores (Figure 3).Conclusion
Preliminary findings suggest that large-scale decoupling of fronto-temporal networks differentiate mdaMCI from healthy controls, that this disconnect is associated with cognitive decline and that this neuronal dysfunction precedes measurable structural neurodegeneration between these two cohorts. Further longitudinal studies investigating the neuronal underpinnings of disease progression will provide insight into potential functional biomarkers and establish the significance of functional decoupling within the sequential model of dynamic biomarkers of the Alzheimer’s pathological cascade.Acknowledgements
No acknowledgement found.References
1. Sperling, R.A., et al., Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia, 2011. 7(3): p. 280-292.
2. Gauthier, S. and J. Touchon, Mild cognitive impairment is not a clinical entity and should not be treated. Archives of Neurology, 2005. 62(7): p. 1164-1166.
3. Klekociuk, S.Z., et al., Reducing false positive diagnosis in MCI: The importance of comprehensive neuropsychological assessment. European Journal of Neurology, 2014. 21: p. 1330-1336.
4. Klekociuk, S.Z. and M.J. Summers, Exploring the validity of Mild Cognitive Impairment (MCI) subtypes: Multiple-domain amnestic MCI is the only identifiable subtype at longitudinal follow up. Journal of Clinical and Experimental Neuropsychology, 2014. 36(3): p. 290-301.
5. Palmer, K., L. Fratiglioni, and B. Winblad, What is mild cognitive impairment? Variations in definitions and evolution of nondemented persons with cognitive impairment. Acta Neurologica Scandinavica, 2003. 107(s179): p. 14-20.
6. Summers, M.J. and N.L.J. Saunders, Neuropsychological measures predict decline to Alzheimer's dementia from mild cognitive impairment. Neuropsychology, 2012. 26(4): p. 498-508.
7. Mars, R.B., et al., On the relationship between the "default mode network" and the "social brain". Front Hum Neurosci, 2012. 6: p. 189.
8. Vincent, J.L., et al., Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol, 2008. 100(6): p. 3328-42.
9. Chen, X., et al., High-order resting-state functional connectivity network for MCI classification. Hum Brain Mapp, 2016. 37(9): p. 3282-96.
10. Reuter, M., H.D. Rosas, and B. Fischl, Highly accurate inverse consistent registration: a robust approach. Neuroimage, 2010. 53(4): p. 1181-96.
11. Whitfield-Gabrieli, S. and A. Nieto-Castanon, Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity, 2012. 2(3): p. 125-141.
Figures
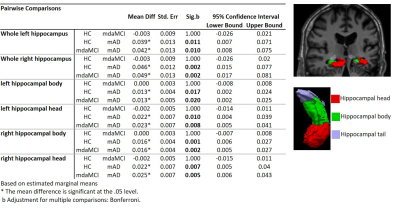
Hippocampal differentiation between groups. Pairwise analysis results for both left and right whole, head and body hippocampal segmentations. Results show significant reduction in all hippocampi segmentation volumes between mAD and mdaMCI and HC. An example of a 3D surface rendering of the left and right hippocampi volumes superimposed over the structural dataset (top right) and individual head, body and tail segmentations (bottom right) are given for reference.
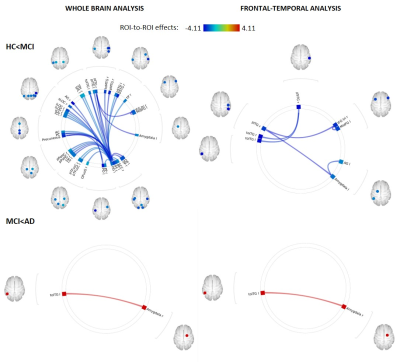
Functional decoupling with disease progression. Whole-brain ROI-to-ROI analysis (top left) revealed a global decoupling in the mdaMCI group with significant reductions within and between temporal, frontal, limbic and occipital regions when compared to HC. A focused analysis including 56 pre-identified frontal, temporal and limbic regions (top right) revealed significant reductions in functional connectivity between the temporal gyrus, amygdala and frontal regions.This decoupling pattern did not continue with disease progression.
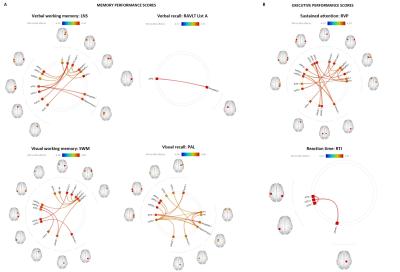
Functional connectivity correlates of memory and executive function performance scores. Bivariate correlation analysis revealed significant association between (A) memory domain scores and fronto-temporal-limbic functional connectivity. Verbal working memory (top left), visual working memory (bottom left) and visual recall (bottom middle). (B) Executive domain: sustained attention performance (top right). Verbal recall analysis and response time (top middle and bottom right) implicated significant associations with temporal-limbic regions only.