1478
Topologically Convergent and Divergent Morphological Grey Matter Networks in Parkinson’s Disease with and without Mild Cognitive Impairment1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, China, 2Psychoradiology Research Unit of Chinese Academy of Medical Sciences (2018RU011), West China Hospital of Sichuan University, Chengdu, China, 3Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati, Cincinnati, OH, United States, 4Department of Neurology, West China Hospital of Sichuan University, Chengdu, China
Synopsis
To use graph theory approaches and high resolution T1-weighted structural MRI to explore the brain grey matter (GM) morphological network in patients with Parkinson's disease (PD) with and without mild cognitive impairment (MCI). The individual morphological brain networks were constructed by estimating interregional similarity in the distribution of regional GM volume of 116 brain regions. The lower path length were found in both patients relative to healthy controls. Altered morphological connection primarily in default mode network were common deficits, while different connectivity characteristic in widespread regions involving temporal and subcortical regions, and cerebellum were observed between the patient groups.
Introduction
Mild cognitive impairment in PD (PD-M) is considered a transitional state between normal cognition (PD-N) and dementia with a prevalence of 15-40% at diagnosis, and is a risk factor for the subsequent development of dementia.1 Cognitive decline poses a significant burden on the patient as well as the caregiver and a better understanding of the underlying pathological processes will aid in directing disease-specific treatment. Recent advances in psychoradiology,2 in conjunction with the graph theory analyses, allow the noninvasive characterization of brain functional and white matter network topologic organization in PD-M.3,4 However, little is known about the individual morphological network in PD-M. Thus, the present study aimed to investigate graph properties of individual morphological brain networks in a sample of early-stage non-demented PD patients.Methods
MRI scanning were carried out in Trio Tim (3T) MR imaging system (Siemens; Erlangen). High resolution T1-weighted structural MRI brain images were obtained from 39 early stage PD patients either with MCI (PD-M, N = 22) or with normal cognition (PD-N, N =17), and 28 demographically-matched healthy controls (HC). Briefly, individual structural images were first segmented into GM, white matter and cerebrospinal fluid. The GM maps were then divided into 116 numbers of brain regions including 90 cortical and subcortical areas and 16 cerebellar areas according to automated anatomical labeling atlas.5 Individual morphological brain networks were constructed by estimating interregional similarity in the distribution of regional GM volume in terms of the Kullback–Leibler divergence measure.6 Graph theory-based global (clustering coefficient Cp, characteristic path length Lp, normalized Cp γ, normalized Lp λ, local efficiency Eloc, global efficiency Eglob, and small-worldness σ) and nodal (nodal efficiency) network measures 7 were calculated. Comparison among the PD-M, PD-N and HC was performed by using analysis of variance followed by pairwise post hoc Fisher’s least significance difference (LSD) tests. To further localize specific pairs of brain regions in which the morphological connection was altered in patients, we used a network-based statistic (NBS) approach. Finally, partial correlations were computed to examine relationships between the network values and cognitive scores (attention and working memory, executive function, language, memory, and visuospatial function) 8 with age, gender, years of education and Unified Parkinson's Disease Rating Scale part III as covariates.Results
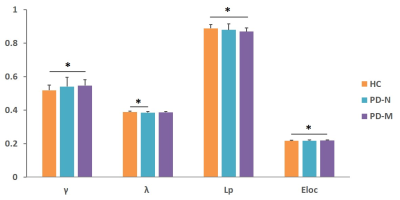
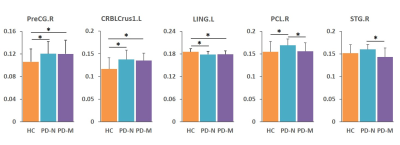
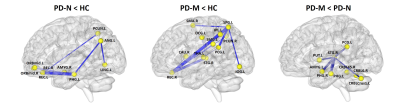
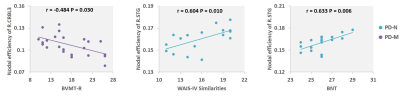
Significantly poorer performance on executive function, memory, and language abilities were observed in patients with PD-M compared to PD-N and HC. Compared with HC, PD-N patients showed a significant decrease in λ, while PD-M showed lower Lp, and higher γ and Eloc (Figure 1). Higher nodal efficiency of right precentral gyrus and left cerebellum Crus1 with lower nodal efficiency of left lingual gyrus were common deficits in both patient groups, and lower nodal efficiency of right paracentral lobule (PCL) and superior temporal gyrus (STG) observed in PD-M than PD-N patients (Figure 2). Decreased connection were similarly found in the default mode network regions of the patient groups relative to HC, while different connection mainly involved in several temporal regions and cerebellum were observed between the patient groups (Figure 3). The nodal efficiency of right cerebellum was negatively correlated with Brief Visuospatial Memory Test–Revised (BVMT-R) score (r = -0.484, P= 0.030) in PD-M group; the nodal efficiency of right STG were positively correlated with Wechsler Adult Intelligence Scale (WAIS-IV) (r = 0.604, P = 0.010) and Boston Naming Test (BNT) (r = 0.633, P = 0.006) scores in PD-N group (Figure 4).Discussion
This study applied graph analysis combined with high resolution T1-weighted structural MRI to assess large-scale brain morphological networks in early stage non-demented PD patients. PD-M showed higher network segregation reflected by higher γ and Eloc, consistent with the previous findings of functional networks,3 providing morphological evidence that higher segregation may be more specific of cognitive impairment in PD. However, the lower Lp in the current study was in the opposite direction from that of white matter networks.4 A possible explanation may be that decreased integration of white matter network might lead to increased GM similarity as a compensation. Patients with PD-M showed lower nodal efficiency in the PCL and STG relative to PD-N, which might be associated with the frequent impairment of frontal executive and temporal language functions in patients with PD-M. Default mode network connectivity disruption might be a common feature in PD, and exhibits a greater extent with increasing levels of cognitive impairment. Moreover, the involvement of the cerebellum is intriguing as recent evidence indicates that the region acts as a critical hub for the control of a wide range of cognitive processes.9 The present study showed a significantly negative correlation of cerebellum with the scores of BVMT-R, indicating that more memory cognitive domain deficit induced more disturbance of the cerebellum. Our findings provided further evidence that disrupted topological organization of large scale complex brain morphological networks contribute to the cognitive impairment in PD patients.Conclusion
Together, this study provides structural evidence for the association of a minority of convergent and a great majority of divergent patterns of morphological brain networks topology between PD-M and PD-N patients, which provides crucial insights into pathophysiological mechanisms of cognition decline of PD.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81621003, 81761128023, 81220108013, 81227002, 81030027), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, grant IRT16R52) of China, the Changjiang Scholar Professorship Award (Award No. T2014190) of China, and the CMB Distinguished a Professorship Award (Award No. F510000/ G16916411) administered by the Institute of International Education.References
1. Yarnall AJ, Rochester L, Burn DJ. Mild cognitive impairment in Parkinson's disease. Age Ageing. 2013;42(5):567-76.
2. Lui S, Zhou XJ, Sweeney JA, et al. Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology. 2016;281(2):357–372.
3. Baggio HC, Sala-Llonch R, Segura B, et al. Functional brain networks and cognitive deficits in Parkinson's disease. Hum Brain Mapp. 2014;35(9):4620-34.
4. Galantucci S, Agosta F, Stefanova E, et al. Structural Brain Connectome and Cognitive Impairment in Parkinson Disease. Radiology. 2017;283(2):515-25.
5. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273-89.
6. Wang H, Jin X, Zhang Y, et al. Single-subject morphological brain networks: connectivity mapping, topological characterization and test-retest reliability. Brain Behav. 2016;6(4):e00448.
7. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069.
8. Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement disorder society task force guidelines. Movement Disorders. 2012;27(3):349–356.
9. Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352-65.
Figures