1477
The Evaluation of Cerebral Blood Flow Deficits in Parkinson’s Disease Dementia using ASL-MRI at 3T1Institute of Biomedical Engineering, Bogazici University, Istanbul, Turkey, 2Department of Electrical and Electronic Engineering, Bogazici University, Istanbul, Turkey, 3Department of Biomedical Engineering, Namik Kemal University, Tekirdağ, Turkey, 4Neuroimaging Unit, Hulusi Behcet Life Sciences Research Center, Istanbul University, Istanbul, Turkey, 5Department of Physiology, Faculty of Medicine, Demiroglu Bilim University, Istanbul, Turkey, 6Department of Neuroscience, Aziz Sancar Institute of Experimental Medicine, Istanbul University, Istanbul, Turkey, 7Department of Psychology, Faculty of Arts and Sciences, Isik University, Istanbul, Turkey, 8Behavioral Neurology and Movement Disorders Unit, Department of Neurology, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey, 9Department of Physiology, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey
Synopsis
This study aims to find cerebral blood flow (CBF) deficits in Parkinson’s disease dementia (PDD). ASL-MRI was utilized to obtain CBF values in PD patients with different stages of cognitive decline at 3T. The CBF values were compared using a whole brain voxel-based analysis. The findings of this study revealed that the PDD group had significant CBF reductions in posterior cerebral areas, mostly in visual cortices, but also in medial parietal areas, and cortical and thalamic components of dorsolateral prefrontal fronto-striatal circuit as compared to both PD group with mild cognitive impairment (PD-MCI) and the cognitively normal PD group (PD-CN).
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder characterized by motor and non-motor deficits (1, 2). Cognitive impairment may be an early manifestation during the disease course, which may progressively worsen to dementia (3). “Mild cognitive impairment” (MCI) is considered as the pre-dementia stage of brain disorders with cognitive impairment (4). Recently formal clinical diagnostic criteria for both MCI (5) and dementia (6) specific to PD were established. Evidence from PD-MCI studies suggest that there may be two subtypes; a more benign, relatively non-progressive subtype, secondary to dopaminergic deafferentation of complex fronto-striatal circuits (7), which is reflected by dysexecutive cognitive findings, and a more malign, progressive subtype with the direct involvement of posterior cortical areas including the visual network and medial parietal components of the episodic memory network, which is reflected with visuospatial, language and memory impairments (8). There is still a need to noninvasively define biomarkers that could predict the status and progression of cognitive impairment in PD. The aim of this study is to determine cerebral blood flow (CBF) deficits in PD at different stages of cognitive decline, including mild cognitive impairment (MCI) and dementia using ASL-MRI.Methods
17 cognitively normal PD (PD-CN), 18 PD with mild cognitive impairment (PD-MCI) and 16 PD with dementia (PDD) patients were included in this study after obtaining their informed consent. Local ethics committee approved our study protocol. The diagnoses were made based on a comprehensive neuropsychological test battery. MDS Task Force Guidelines for the diagnoses of PD dementia and PD MCI were used. PD patients with multiple cognitive deficits and functional impairment were identified as PDD, and those with no functional impairment but having an Addenbrooke’s Cognitive Examination-Revised (ACE-R) score < 83, were identified as PD-MCI by expert behavioral neurologists. The remaining PD patients were labeled as PD-CN. The participants were scanned on a 3 T Philips MRI scanner with a 32-channel head coil. ASL-MR images were acquired from six slices using eight different inversion times (TIs) and with 30 averages. CBF maps were calculated using an in-house program written in MATLAB R2018a (MathWorks Inc., Natick, MA). The CBF maps, which were co-registered to T1-weighted MRI, were registered to MNI152 brain atlas using FMRIB Software Library (FSL). Whole-brain voxel-based CBF comparison between patient groups was performed using statistical parametric mapping (SPM) 12 software, and age was used as a covariate. The level of statistical significance was set to uncorrected cluster forming threshold of P < 0.005 and cluster level family-wise error (FWE) correction threshold of P < 0.05, with a minimum cluster size of 50 voxels.Results
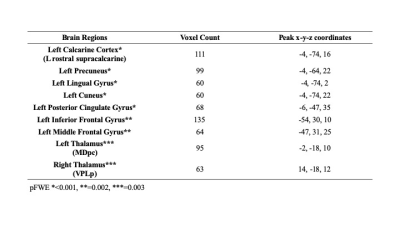
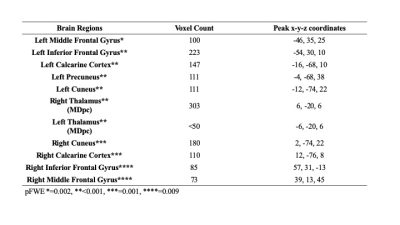
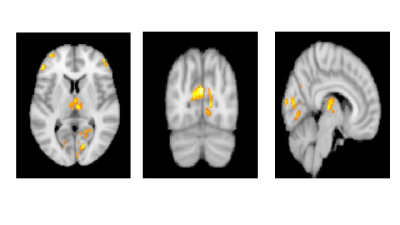
The mean age of PD-CN, PD-MCI and PDD patients were 60±10, 63±9, and 71±6, respectively. The CBF values of PDD was significantly lower than that of PD-MCI in bilateral thalami (left parvocellular mediodorsal nucleus and right posterior ventroposterolateral nucleus), several posterior cortical areas in the left hemisphere including four different clusters located in the primary visual cortex, dorsal and ventral extrastriate visual cortices, and posterior cingulate cortex. In addition to these areas, two different clusters in left anterior areas also had decreased perfusion in PDD than PD-MCI, one in inferior frontal gyrus and the other in middle frontal gyrus (pFWE < 0.003) (Table 1). PDD versus PD-CN comparison revealed that perfusion reduction in four visual clusters distributed evenly in two hemispheres and encompassing primary visual cortex and extrastriate areas. Four anteriorly located clusters, which were also different between PDD and PD-CN, were evenly distributed in bilateral inferior and middle frontal gyri. The blood flow of bilateral parvocellular mediodorsal nucleus of thalamus was also reduced in patients with PDD than PD-CN (pFWE <0.002). (Table 2). On the other hand, there was no CBF difference between PD-CN and PD-MCI.Discussion
In this study, we demonstrated that PDD patients had reduced perfusion in the frontal and posterior medial parietal and occipital lobes compared to PD-MCI and PD-CN patients. The results of our study were consistent with previous studies in which PDD had been characterized by posterior hypoperfusion affecting primarily precuneus, posterior cingulate, and occipital regions (10, 11). The underlying mechanisms of the reduction of perfusions in posterior cortical regions remains controversial. However, it has been thought that posterior abnormality causes visuospatial and memory dysfunctions in patients with PDD (12). In addition to classical posterior hypoperfusion, probably denoting the cortical involvement in PD neurodegeneration process, there were also anteriorly and subcortically located clusters, which are likely to be the components of dorsolateral prefronto-striatal circuit that has a nigro-striatal dopaminergic input. However, we did not find any CBF differences between PD-CN and PD-MCI groups probably reflecting the sensitivity of ASL-MRI in revealing the subtler CBF differences that might underlie this milder form of cognitive impairment.Conclusion
In conclusion, dementia stage in PD could be separated from mild cognitive impairment and cognitively normal stages with CBF deficits in non-dopaminergically-mediated posterior and dopaminergically-mediated frontal networks using ASL-MRI technique; which is in line with the dual syndrome hypothesis of PD cognitive impairment.Acknowledgements
This study was supported by TUBITAK 1001 project #115S219 and Istanbul University Scientific Research Projects Unit project #1567/42362.References
1. Wright Willis A,
2. Jankovic J. Parkinson's disease: clinical features and diagnosis. J
3. Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed
4. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004
5. Litvan I, Goldman JG, Troster AI,
6. Emre M,
7. Alexander GE,
8. Martinez-Horta S,
9. Jack CR, Jr.,
10. Garcia-Garcia D, Clavero P, Gasca Salas C, Lamet I, Arbizu J, Gonzalez-Redondo R, et al. Posterior
11. Tang Y, Ge J, Liu F, Wu P,
12.