1470
In-vivo classification of arteriolar sclerosis and small vessel atherosclerosis in aging, and prediction of cognitive decline1Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States
Synopsis
Arteriolar sclerosis and small vessel atherosclerosis are two age-related neuropathologies that are common in older adults and have been linked to cognitive decline and dementia. Definitive diagnosis of either pathology is only possible at autopsy. In this work, a novel MRI-based classifier was developed for predicting the presence of arteriolar sclerosis or small vessel atherosclerosis in-vivo. The classifier was first developed based on ex-vivo MRI and pathology data from a large community-based cohort of older adults and was then translated in-vivo. The performance of the classifier was assessed both ex-vivo and in-vivo.
Introduction
Arteriolar sclerosis and small vessel atherosclerosis are two age-related neuropathologies that are common in older adults and have been linked to cognitive decline and dementia1. Definitive diagnosis of either pathology is only possible at autopsy. The purpose of this work was to develop a model that predicts the presence of arteriolar sclerosis or atherosclerosis based on in-vivo MRI and basic demographic features. The model was first developed based on ex-vivo MRI and pathology data from a large community-based cohort of older adults and was then translated in-vivo. The performance of the model was assessed both ex-vivo and in-vivo.Methods
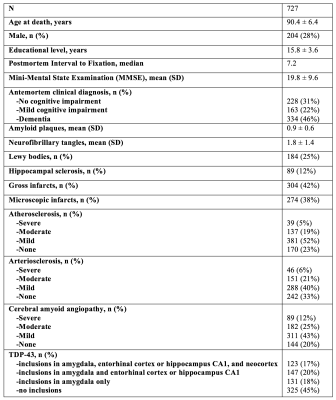
Postmortem dataCerebral hemispheres were obtained from 727 participants of the Rush Memory and Aging Project2 (MAP) and Religious Orders Study3 (ROS), two longitudinal cohort studies of aging (Fig.1). A brain hemisphere from each participant was imaged ex-vivo on a clinical 3T MRI scanner, while immersed in 4% formaldehyde solution. Following ex-vivo MRI, all hemispheres underwent detailed neuropathologic examination by a board-certified neuropathologist blinded to clinical and imaging findings. The volume of white matter hyperintensities (WMH) per lobe and regional white matter R2 values were extracted from the ex-vivo MRI data of each participant.
Development of the ex-vivo MRI classifier
Due to the high number and different nature of features, separate models were first developed for pathology prediction based on lobar WMH volumes and regional white matter R2, each generating a separate risk score. For example, the WMH risk score per person was calculated by training a random forest classifier based exclusively on lobar WMH features. This classifier outputs a risk score for pathology, where higher values indicate a higher chance of having the pathology, and vice versa. A similar approach was used for regional R2 values. The resulting risk scores were then used along with demographics (age, sex, and education) as features in a logistic regression classifier. Performance evaluation involved 100 repeats of stratified shuffle split cross-validation with 80% of the data used for training, and 20% for testing. The separate models based on WMH or regional R2, and the final logistic regression model were developed and tuned in every training fold of the cross-validation. We also developed a model based exclusively on the total volume of WMH, which is generally considered as a marker of small vessel disease4,5 (but is not specific to any small vessel pathology). The performance of the two models was compared using a Wilcoxon signed rank test (p<0.05).
In-vivo translation of the classifier
Both in-vivo and ex-vivo 3T MRI data were available on 17 participants. Lobar WMH and regional R2 values were extracted from the in-vivo and ex-vivo MRI data and linear mixed-effects models were used to establish the relationship between the in-vivo and ex-vivo values. These models were used to achieve in-vivo translation of the classifier.
Testing the classifier in-vivo
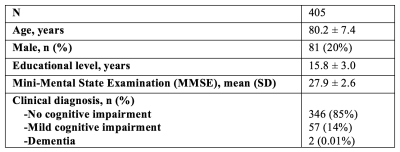
In-vivo 3T MRI and longitudinal cognition data were obtained on 405 MAP and ROS participants, different than those used in the development of the classifier (Fig.2). An in-vivo classification confidence score was generated for each participant, expressing the likelihood that a participant imaged in-vivo suffered by either arteriolar sclerosis or small vessel atherosclerosis. Since pathology information was not available for this cohort of older adults, the performance of the classifier in-vivo was assessed by testing the association of the classification confidence score with the change in global cognition two years after baseline MRI, using Pearson’s correlation. The same analysis was repeated for five cognitive domains: semantic memory, episodic memory, working memory, perceptual speed, and visuospatial abilities.
Discussion
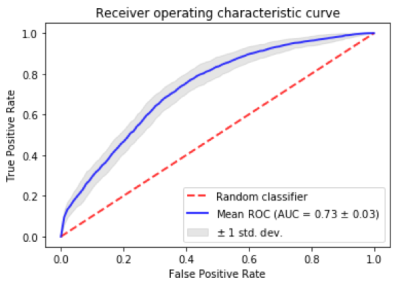
A novel classifier of arteriolar sclerosis or small vessel atherosclerosis, two important age-related neuropathologies that can be diagnosed only at autopsy, was developed in this work. The performance of the ex-vivo classifier was far superior than chance (AUC=0.5) which represents the current lack of a diagnostic test. The performance of the ex-vivo classifier was also superior to that of a classifier based exclusively on WMH information, which is thought to represent small vessel disease in general and is not specific to arteriolar sclerosis or atherosclerosis. Furthermore, in-vivo testing in a large community cohort showed that the new classifier was associated with cognitive decline two years after baseline MRI, and that this decline was specifically in cognitive domains that have previously been linked to arteriolar sclerosis and atherosclerosis1. The above demonstrate the high clinical relevance and significance of the new classifier.Conclusion
A novel MRI-based classifier was developed for predicting the presence of arteriolar sclerosis or small vessel atherosclerosis in-vivo. This classifier may have broad clinical implications including refined participant selection and enhanced monitoring of the treatment response in clinical drug and prevention trials.Acknowledgements
This study was supported by National Institutes of Health grants P30AG010161, UH2NS100599, UH3NS100599, R01AG064233, R01AG17917.References
1. Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of Cerebral Vessel Disease to Alzheimer’s Disease Dementia and Cognitive Function in Older Persons: A Cross-sectional Study. The Lancet Neurology. 2016;15(9):934-943.
2. Bennett DA, Schneider JA, Buchman AS, et al. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663.
3. Bennett DA, Wilson RS, Arvanitakis Z, et al. Selected findings from the Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 2013;33 Suppl 1:S397-403.
4. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta- analysis. BMJ 2010;341:c3666.
5. Erten-Lyons D, Dodge HH, Woltjer R, et al. Neuropathologic basis of age-associated brain atrophy. Neurology 2013;70:616-622.