1468
Diffusion Kurtosis Imaging of Basal Ganglia and Thalamus: Compare between Alzheimer’s Disease, Major Depressive Disorder and Healthy Subjects1Radiology, Faculty of Medicine Siriraj Hospital, Bangkok, Thailand, 2Geriatric Medicine, Faculty of Medicine Siriraj Hospital, Bangkok, Thailand, 3Psychiatry, Faculty of Medicine Siriraj Hospital, Bangkok, Thailand
Synopsis
The brain circuit pathways of major depressive disorder (MDD) and Alzheimer’s disease (AD) could overlap. The authors hypothesized that AD and MDD subjects would have different diffusion parameters compared to healthy elderly controls (HE). Forty-seven age-matched subjects (AD=19, MDD=11, HE=20) were enrolled and performed MRI included structural brain imaging and diffusion kurtosis imaging (DKI) and then diffusional parameters were compared to differentiate between the three groups. Caudate nucleus and thalamus revealed significant different of diffusion parameters between AD and HE, and between AD and MDD but no significant different between MDD and HE. Diffusion imaging can demonstrate microstructural change in AD.
Introduction
Diffusion kurtosis imaging (DKI) is an emerging MRI technique for evaluating non-gaussian diffusion of water molecules as representing tissue heterogeneity in the brain. It can detect isotropic diffusion of water in the neurons. The decrease of kurtosis parameter could be a sign of neuronal loss. Subcortical nuclei such as basal ganglia and thalamus are involved in cognitive function and also correlate with mood circuit. Hence, Alzheimer’s disease (AD) and major depressive disorder (MDD) may show alteration of the microstructure at these subcortical nuclei. DKI may play a role to detect this change.Purpose
To compare and evaluate abnormalities in basal ganglia and thalamus in AD and MDD patients comparing to healthy elderly subjects by using diffusion imaging.Methods
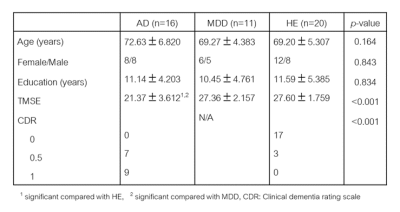
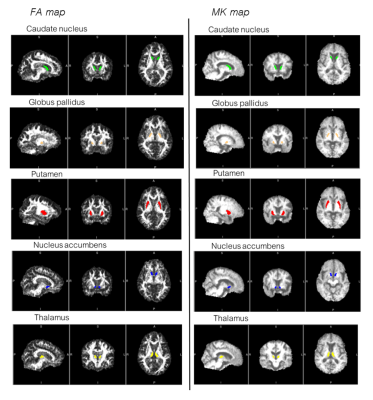
Forty-seven age-matched subjects (AD=19, MDD=11, healthy elderly (HE) =20) were included. The MRI protocol included routine structural brain imaging and DKI. Diffusion tensor and kurtosis parameters were calculated-using FSL software. The parametric maps of subcortical nuclei were obtained including FA (fractional anisotropy), MD (mean diffusivity), RD (radial diffusivity), AxD (axial diffusivity), MK (mean kurtosis), RK (radial kurtosis), AK (axial kurtosis) and MKT (mean kurtosis tensor). Statistical analysis of each diffusional parameter was compared to differentiate between the three groups.Results
Comparison between AD and HEAlmost all diffusion tensor parameters in the right and left caudate nuclei (except FA) in AD showed significantly higher value than those of HE. Only the right caudate nucleus showed significant decreased FA value in AD as compared with HE. All of the diffusion kurtosis parameters in the right and left caudate nuclei in AD were significantly lower value than those of HE. The right and left thalami revealed significantly lower FA, higher MD, AxD, RD, and lower MK, AK, RK, MKT values in AD than those of HE. The right and left globus pallidus in AD revealed significantly higher value of FA than those of HE. The right and left nucleus accumbens in AD demonstrated significantly higher diffusion tensor parameters (MD, AxD, RD) than those of HE. Only the right nucleus accumbens showed decreased FA in AD. There was no significant difference of the diffusion tensor or kurtosis parameters in both sided putamen between AD and HE. However, there was tendency of higher values of FA and kurtosis parameters and lower values of MD, AxD and RD in AD than that of HE.
Comparison between AD and MDD
The right and left caudate nuclei and thalami showed significantly higher values of MD, AxD, RD and lower diffusion kurtosis parameters in AD than those of MDD. The right and left globus pallidus in AD showed significantly higher value of FA than those of MDD. But only right globus pallidus revealed decreased RD and increased RK comparing AD and MDD. The right nucleus accumbens showed significant lower FA and higher MD, AxD, RD values in AD than those of MDD. Only RD of the left nucleus accumbens in AD showed significantly higher value as compared with MDD. There was no significant difference of diffusion tensor or kurtosis parameters in both sided putamen between AD and MDD.
Comparison between MDD and HE
There was no any significant difference of all diffusion tensor or kurtosis parameters in all subcortical nuclei between MDD and HE.
Discussion
Both the right and left caudate nuclei revealed significantly increased almost diffusion tensor parameters (MD, AxD, RD) while diffusion kurtosis parameters (MK, AK, RK, MKT) also significantly decreased value in AD compared to HE and MDD groups. Previous study reported increase of diffusion parameter, especially MD, in the caudate nucleus in AD patients, could be resulted from loss of neuron and increased in extracellular free diffusion space1. While decreasing diffusion kurtosis parameters, such as MK, might be resulted from reduction of neurons and fibers including loss of caudate nucleus volume1. All diffusion tensor and almost all diffusion kurtosis parameters on both thalami showed significant difference in AD compared to HE and MDD. Similar to the result of caudate nucleus, increased diffusivity and decreased kurtosis represent neuronal loss2. On both putamen and globus pallidus, the diffusion tensor and kurtosis parameters showed conversely to the other regions. The reason of this result was described that Amyloid β accumulated in the lentiform nucleus in AD patients, similar to Yuan et al2 and Wang et al1. Few DKI studies in the APP/PS1 transgenic mice supported that Amyloid β accumulation in mice’s brain revealed increased MK in many brain regions3,4. Many studies revealed significant volume reduction of basal ganglia and thalamus in depression patients5-7. The authors expected increased diffusivity or decreased kurtosis values. On the contrary, the results of our study showed no significant difference between MDD and HE groups. It might be uncertain onset and duration of the disease in our subjects that might not alter in the microstructure of the brain. Another reason is that pathophysiology of depression is abnormality in circuit of the brain8, whereas synapse or neurotransmitter which is not change the microstructure or anatomy.Conclusion
AD has microstructural change at subcortical nuclei, revealed by decreased diffusion kurtosis parameters whereas MDD and healthy controls revealed no significant difference of microstructure at the subcortical nuclei.Acknowledgements
The authors would like to thank Mrs. Angkana Jongsawaddipatana (for subject recruitment), Mrs Supattra Junkhum (for neuropsychological testing), Miss Pusanisa Yangyoo (for neuropsychological testing), and Dr. Masuma Tetcharoenpanit (for proposal preparation).
The authors would like to thank Ms.Panida Charnchaowanish for raw data transferring and Mr.Suthipol Udompunthurak for statistic advice.
References
1. Wang ML, Wei XE, Fu JL, Li W, Yu MM, Li PY, et al. Subcortical nuclei in Alzheimer's disease: a volumetric and diffusion kurtosis imaging study. Acta radiologica. 2018;59(11):1365-71.
2. Yuan L, Sun M, Chen Y, Long M, Zhao X, Yin J, et al. Non-Gaussian diffusion alterations on diffusion kurtosis imaging in patients with early Alzheimer's disease. Neuroscience letters. 2016;616:11-8. 3. Vanhoutte G, Pereson S, Delgado YPR, Guns PJ, Asselbergh B, Veraart J, et al. Diffusion kurtosis imaging to detect amyloidosis in an APP/PS1 mouse model for Alzheimer's disease. Magnetic resonance in medicine. 2013;69(4):1115-21.
4. Praet J, Manyakov NV, Muchene L, Mai Z, Terzopoulos V, de Backer S, et al. Diffusion kurtosis imaging allows the early detection and longitudinal follow-up of amyloid-beta-induced pathology. Alzheimer's research & therapy. 2018;10(1):1.
5. Lacerda AL, Nicoletti MA, Brambilla P, Sassi RB, Mallinger AG, Frank E, et al. Anatomical MRI study of basal ganglia in major depressive disorder. Psychiatry research. 2003;124(3):129-40.
6. Nugent AC, Davis RM, Zarate CA, Jr., Drevets WC. Reduced thalamic volumes in major depressive disorder. Psychiatry research. 2013;213(3):179-85.
7. Zhao L, Wang Y, Jia Y, Zhong S, Sun Y, Zhou Z, et al. Microstructural Abnormalities of Basal Ganglia and Thalamus in Bipolar and Unipolar Disorders: A Diffusion Kurtosis and Perfusion Imaging Study. Psychiatry investigation. 2017;14(4):471-82.
8. Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12(9):3628-41.