1467
Quantitative susceptibility and R2* mapping in mild cognitive impairment and early Alzheimer’s disease1School of Psychology and Centre for Brain Research, University of Auckland, Auckland, New Zealand, 2Brain Research New Zealand, Auckland, New Zealand, 3Department of Physics, University of Auckland, Auckland, New Zealand, 4Institute of Psychiatry, Psychology & Neuroscience, King's College London, London, United Kingdom, 5University of Otago, Christchurch, New Zealand, 6Brain Reserach New Zealand, Christchurch, New Zealand
Synopsis
There is a growing body of work (post-mortem and in vivo) to support the link between iron and the pathophysiology of neurodegenerative disorders. While much of the current literature has focused on later stages of diseases such as Parkinson’s and Alzheimer’s disease (AD), how iron may contribute to early pathology is less well understood. We employed QSM and R2* mapping in a group of mild cognitive impairment and early AD subjects along with age-matched controls. Our pilot results suggest that magnetic susceptibility is higher in the cortex of the cognitively impaired group and is negatively correlated with cognition.
Background
In the brain, iron is crucial for maintaining the high metabolic and energetic requirements of neuronal tissue, neurotransmitter synthesis and myelin production. If the fine homeostatic balance of iron is disrupted, excessive amounts catalyse the formation of damaging free radicals (1). Observations of iron in pathological post-mortem tissue have provided a growing body of evidence to support the link between iron metabolism and the pathophysiology of neurodegenerative disorders (2,3), including multiple sclerosis(4,5), Parkinson’s disease (6–9) and Alzheimer’s disease (AD) (10–13). Given the different magnetic susceptibilities of paramagnetic iron deposits and diamagnetic brain tissue, quantitative susceptibility mapping (QSM) has been used to study brain iron in vivo in normal ageing (14–16), AD (17–19) and mild cognitive impairment (MCI) (20–23), indicating iron (as measured with QSM), maybe an early indicator of AD pathology. However, of the aforementioned MCI studies, one was at 7T (not commonplace), and one in subcortical vascular MCI. Thus we sought to apply QSM in MCI and early AD at 3T to investigate 1) its ability to detect early changes in iron deposition, and 2) how susceptibility values vary with cognition. Using a multi-echo protocol for QSM, allowed simultaneous R2* mapping from the magnitude data, which we also investigated, given previous reports on its utility in studying iron deposition (24,25).Methods
Subjects enrolled in the longitudinal, Brain Research New Zealand, Dementia Prevention Research Clinic in Auckland were invited to take part. Subjects were classified following a multi-disciplinary assessment, including the Addenbrooke’s Cognitive Examination-III (ACE-III), detailed neuropsychology and neuroimaging. Recruitment is ongoing, but to date 21 subjects have been scanned and include 3 AD subjects and 11 MCI subjects; grouped for statistical testing into a “cognitively impaired” (CI) group (mean age +/- SD 74 +/- 9.4 years, 8 females); and 7 age matched controls the “cognitively normal” (CN) group (mean age +/- SD 75 +/- 8.3 years, 6 females).Imaging was performed using a MAGNETOM Skyra 3T MR scanner (Siemens Healthcare, Erlangen, Germany), using a 32-channel head coil. Phase-sensitive data for QSM were collected with a 3D gradient echo sequence, eight echoes, TE1=5.67 ms, ∆TE=5.45ms, TR=48ms, flip angle=20 degrees, field-of-view=280x280x144 mm, voxel size=1.0x1.0x2.0 mm3 and receiver bandwidth=250 Hz/pixel. The Morphology Enabled Dipole Inversion (MEDI) toolbox (26) was used, with options of graph cuts for phase unwrapping (27) and projection onto dipole field (PDF) (28) to remove background field. MEDI+0 (26) was used to solve for magnetic susceptibility in the brain relative to cerebrospinal fluid. R2* maps were also generated using the MEDI toolbox.
Relative susceptibility and R2* maps for all subjects were registered to the same template (MNI152 2mm template) using the FMRIB Software Library (FSL) registration tools (29). Regions of interest investigated were: the iron-rich globus pallidus and putamen, the hippocampus, because of its relevance to memory and AD pathology, and the cortical regions of the posterior cingulate cortex (PCC) and precuneus, given the findings of Kim et al. (22). Finally, cortical grey matter was selected as a ROI, to investigate the utility of a large holistic region in assessing iron load. All structures were defined using the Harvard-Oxford probabilistic atlas. In each ROI, group differences (CN vs CI) in relative susceptibility and R2* were investigated using t-tests; correlation with ACE-III was tested using Spearman’s rank correlation co-efficient.
Results
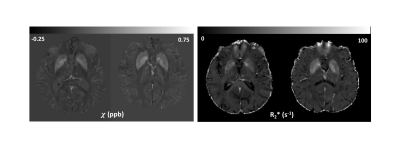
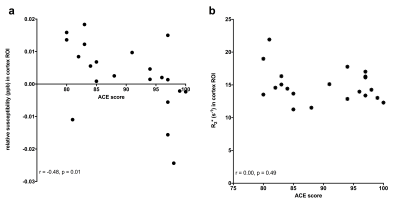
Representative relative susceptibility and R2* maps are shown in figure 1, clearly depicting higher values in the pallidum and putamen as expected. Relative susceptibility maps show good white matter and cortical grey contrast. Age was not significantly different between the CN and CI groups (p=0.98). While relative susceptibility was consistently higher in the cortex and hippocampus in the cognitively impaired group, only the composite cortical ROI reached significance (p=0.03). We observed no evidence of a group difference in R2* in any ROI (p>0.05 in all). Relative susceptibility was negatively correlated with ACE-III (a global cognitive score) in the cortex (Spearman’s rho = -0.48, p=0.01, figure 3), and hippocampus (Spearman’s rho = -0.60, p=0.002), but not significantly correlated in the other ROIs. No correlations with R2* and ACE-III were found in any of the ROIs.Discussion and Conclusion
Our preliminary results indicate that relative susceptibility is higher in the cortex in CI vs CN participants, which suggests increased regional iron concentration. Based on our results, QSM is more sensitive at detecting iron differences (regionally and between groups) than R2*.Unlike other neurodegenerative diseases where specific loci of iron deposits have been reported (19,30) e.g. in the deep grey basal ganglia structures, our results suggest more widespread cortical areas might be more involved, at least in cognitive decline of the Alzheimer type. It has been suggested that cortical iron is co-localised with accumulation of amyloid β protein (22,31), which we will test in our larger (to be recruited) cohort with additional amyloid PET imaging. However, in cortical regions it can be difficult to map susceptibility accurately since the outer edge of the cortex has neighbouring voxels without reliable phase information that can be used for background field removal. This warrants further investigation, using new QSM algorithms that maybe be better at preserving cortical regions (32).
Acknowledgements
This work was funded by Brain Research New Zealand.
We would like to thank Kieran O’Brien, Siemens Healthcare Pty. Ltd, Australia, for useful discussions on QSM acquisition and processing.
We would like to thank Jane Govender from the Dementia Prevention Research Clinic for all her help with recruitment, and the MR technologists at the Centre for Advanced MRI (CAMRI) for participant scanning.
References
1. Kehrer JP. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology. 2000 Aug 14;149(1):43–50.
2. Gerlach M, Ben-Shachar D, Riederer P, Youdim MBH. Altered Brain Metabolism of Iron as a Cause of Neurodegenerative Diseases? Journal of Neurochemistry. 1994 Sep 1;63(3):793–807.
3. Ke Y, Ming Qian Z. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2(4):246–53.
4. Craelius W, Migdal MW, Luessenhop CP, Sugar A, Mihalakis I. Iron deposits surrounding multiple sclerosis plaques. Arch Pathol Lab Med. 1982;106(8):397–9.
5. LeVine SM. Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res. 1997;760(1–2):298–303.
6. Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114 ( Pt 4:1953–75.
7. Good PF, Olanow CW, Perl DP. Neuromelanin-containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson’s disease: a LAMMA study. Brain Res. 1992;593(2):343–6.
8. Popescu BF, George MJ, Bergmann U, Garachtchenko AV, Kelly ME, McCrea RP, et al. Mapping metals in Parkinson’s and normal brain using rapid-scanning x-ray fluorescence. Phys Med Biol. 2009;54(3):651–63.
9. Gardner B, Dieriks BV, Cameron S, Mendis LHS, Turner C, Faull RLM, et al. Metal concentrations and distributions in the human olfactory bulb in Parkinson’s disease. Sci Rep. 2017 Sep 5;7(1):10454.
10. Grundke-Iqbal I, Fleming J, Tung YC, Lassmann H, Iqbal K, Joshi JG. Ferritin is a component of the neuritic (senile) plaque in Alzheimer dementia. Acta Neuropathol. 1990;81(2):105–10.
11. Morris CM, Kerwin JM, Edwardson JA. Non-haem iron histochemistry of the normal and Alzheimer’s disease hippocampus. Neurodegeneration. 1994;3(4):267–75.
12. Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci. 1998;158(1):47–52.
13. Hare DJ, Raven EP, Roberts BR, Bogeski M, Portbury SD, McLean CA, et al. Laser ablation-inductively coupled plasma-mass spectrometry imaging of white and gray matter iron distribution in Alzheimer’s disease frontal cortex. NeuroImage. 2016 Aug 15;137:124–31.
14. Bilgic B, Pfefferbaum A, Rohlfing T, Sullivan EV, Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. NeuroImage. 2012;59(3):2625–35.
15. Betts MJ, Acosta-Cabronero J, Cardenas-Blanco A, Nestor PJ, Düzel E. High-resolution characterisation of the aging brain using simultaneous quantitative susceptibility mapping (QSM) and R2* measurements at 7 T. NeuroImage. 2016;138:43–63.
16. van Bergen JMG, Li X, Quevenco FC, Gietl AF, Treyer V, Meyer R, et al. Simultaneous quantitative susceptibility mapping and Flutemetamol-PET suggests local correlation of iron and β-amyloid as an indicator of cognitive performance at high age. NeuroImage. 2018 Jul 1;174:308–16.
17. Acosta-Cabronero J, Williams GB, Cardenas-Blanco A, Arnold RJ, Lupson V, Nestor PJ. In Vivo Quantitative Susceptibility Mapping (QSM) in Alzheimer’s Disease. PLoS One [Internet]. 2013 Nov 21 [cited 2017 Aug 17];8(11). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3836742/
18. Moon Y, Han S-H, Moon W-J. Patterns of Brain Iron Accumulation in Vascular Dementia and Alzheimer’s Dementia Using Quantitative Susceptibility Mapping Imaging. Journal of Alzheimer’s Disease. 2016 Jan 1;51(3):737–45.
19. Du L, Zhao Z, Cui A, Zhu Y, Zhang L, Liu J, et al. Increased Iron Deposition on Brain Quantitative Susceptibility Mapping Correlates with Decreased Cognitive Function in Alzheimer’s Disease. ACS Chem Neurosci. 2018 Jul 18;9(7):1849–57.
20. van Bergen JMG, Li X, Hua J, Schreiner SJ, Steininger SC, Quevenco FC, et al. Colocalization of cerebral iron with Amyloid beta in Mild Cognitive Impairment. Sci Rep [Internet]. 2016 Oct 17 [cited 2017 Aug 14];6. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5066274/
21. Sun Y, Ge X, Han X, Cao W, Wang Y, Ding W, et al. Characterizing Brain Iron Deposition in Patients with Subcortical Vascular Mild Cognitive Impairment Using Quantitative Susceptibility Mapping: A Potential Biomarker. Front Aging Neurosci [Internet]. 2017 Mar 30 [cited 2017 Aug 17];9. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5371674/
22. Kim H-G, Park S, Rhee HY, Lee KM, Ryu C-W, Rhee SJ, et al. Quantitative susceptibility mapping to evaluate the early stage of Alzheimer’s disease. NeuroImage: Clinical. 2017;16:429–38.
23. Ayton S, Fazlollahi A, Bourgeat P, Raniga P, Ng A, Lim YY, et al. Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain. 2017 Aug 1;140(8):2112–9.
24. Feng X, Deistung A, Reichenbach JR. Quantitative susceptibility mapping (QSM) and R2* in the human brain at 3T. Zeitschrift für Medizinische Physik [Internet]. 2017 Jun 7 [cited 2017 Aug 14]; Available from: http://www.sciencedirect.com/science/article/pii/S0939388916301143
25. Yan S-Q, Sun J-Z, Yan Y-Q, Wang H, Lou M. Evaluation of Brain Iron Content Based on Magnetic Resonance Imaging (MRI): Comparison among Phase Value, R2* and Magnitude Signal Intensity. PLoS One [Internet]. 2012 Feb 20 [cited 2019 Nov 5];7(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3282752/
26. Liu Z, Spincemaille P, Yao Y, Zhang Y, Wang Y. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping: QSM With Automatic Uniform CSF Zero Reference. Magn Reson Med. 2018 May;79(5):2795–803.
27. Dong J, Liu T, Chen F, Zhou D, Dimov A, Raj A, et al. Simultaneous phase unwrapping and removal of chemical shift (SPURS) using graph cuts: application in quantitative susceptibility mapping. IEEE Trans Med Imaging. 2015 Feb;34(2):531–40.
28. Liu T, Khalidov I, de Rochefort L, Spincemaille P, Liu J, Tsiouris AJ, et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed. 2011 Nov;24(9):1129–36.
29. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208--19.
30. Uchida Y, Kan H, Sakurai K, Arai N, Kato D, Kawashima S, et al. Voxel-based quantitative susceptibility mapping in Parkinson’s disease with mild cognitive impairment. Movement Disorders. 2019;34(8):1164–73.
31. Gong N-J, Dibb R, Bulk M, van der Weerd L, Liu C. Imaging beta amyloid aggregation and iron accumulation in Alzheimer’s disease using quantitative susceptibility mapping MRI. NeuroImage. 2019 May 1;191:176–85.
32. Bollmann S, Rasmussen KGB, Kristensen M, Blendal RG, Østergaard LR, Plocharski M, et al. DeepQSM - using deep learning to solve the dipole inversion for quantitative susceptibility mapping. NeuroImage. 2019 Jul 15;195:373–83.
Figures