1464
Perfusion Imaging of Patients with Alzheimer’s Disease by Using highly-accelerated Spin and Gradient Echo (SAGE) DSC-MRI
Yi-Fen Yen1, Mary Kate Manhard1, Annie G. Bryant2, Rachel E. Bennett2, Kimberly A. Stephens1, David H. Salat1,3, Keith A. Johnson2, Bradley T. Hyman2, Kawin Setsompop1, and Susie Huang1
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Neurology, Massachusetts General Hospital, Boston, MA, United States, 3VA Boston Healthcare System, Neuroimaging Research for Veterans Center, Boston, MA, United States
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Neurology, Massachusetts General Hospital, Boston, MA, United States, 3VA Boston Healthcare System, Neuroimaging Research for Veterans Center, Boston, MA, United States
Synopsis
We have identified reduced cerebral blood flow, abnormally long mean transit time, and large capillary transit time heterogeneity in seven individuals with mild cognitive impairment or Alzheimer’s disease as compared to healthy subjects by using a highly accelerated dynamic susceptibility enhanced (DSC) MRI technique. This perfusion imaging technique provides whole brain coverage using simultaneous multi-slice acquisition and collects spin and gradient echo in one dynamic scan. The spin-echo and gradient-echo DSC-MRI acquisition enables probing and potentially distinguishing micro- and macro-vascular contributions to perfusion in older adults using a single injection of gadolinium.
INTRODUCTION
Vascular dysfunction is increasingly recognized as a key contributor to the development of Alzheimer’s disease (AD) (1-5). Decrease in CBF is an early indicator of AD (2,4,6). DSC-MRI in AD shows prolonged mean transit time (MTT) and large capillary transit time heterogeneity (CTH) that correlate with symptom severity in AD, possibly due to capillary dysfunction, blood vessel abnormalities, and blood-brain barrier breakdown (7-8). Gradient-echo (GE) signals are highly susceptibility-sensitive and dominated by both microscopic and macroscopic vessels, whereas spin-echo (SE) signals are predominantly sensitive to microscopic vessels such as capillaries (radius < 10 µm) (9-12). Acquiring both SE and GE (SAGE) DSC data will enable probing and distinguishing micro- and macro-vascular contributions to perfusion. However, current DSC-MRI techniques require two separate MR acquisitions to obtain SAGE DSC data with only partial brain coverage and require two injections of Gd contrast agent. We have developed a novel SAGE DSC-MRI technique to obtain SE and GE data in one acquisition with high acceleration for whole brain coverage without compromising temporal resolution (13). Here, we use this technique to probe cerebrovascular function alongside high-sensitivity PET imaging markers of tau in patients with AD and mild cognitive impairment (MCI) and compared to DSC-MRI data from cognitively healthy adults.METHODS
Seven patients (age 66-85, 3F/4M) with MCI and AD as well as three healthy subjects (age 23-34, 2F/1M) were scanned on a Siemens 3T Prisma MRI system. T1-weighted (MPRAGE) imaging was acquired followed by SAGE DSC-MRI with a bolus injection of 0.1 mmol/kg Dotarem® (gadoterate meglumine by Guerbet LLC, France) followed by 20 mL saline flush both at 5 mL/s injection rate. The SAGE DSC-MRI (13) parameters are: 2.4 mm in-plane resolution, thirty-three 3 mm thick slices and a 15% slice gap; TR 1500 ms; GE at TE 30 ms; SE at TE 90 ms; 244 time series; temporal resolution 1.5 seconds. In addition, we also included DSC-MRI data from another cohort of six healthy adults (age 61-71, 5F/1M) whose age range better matches that of the seven patients. Only GE DSC-MRI was acquired on these older healthy subjects but the imaging parameters were identical to the GE portion of the patient DSC data.Motion correction was performed using FSL (14). Perfusion analysis was performed with PGUI software (15) to derive parametric perfusion images. MTT was computed via the central volume theorem by using CBV from the integration of the dynamic curve and CBF from residue function after singular value decomposition of the arterial input function. CTH was derived in PGUI based on a previously developed vascular model (16-19) as the standard deviation of the transit time distribution calculated as the time derivative of the residue function.
Parametric perfusion images were registered to the MPRAGE images by using a temporal mean of the SE-DSC time series as a reference. The MPRAGE images were segmented using FreeSurfer (20-23). Pairwise relative comparisons in the mean perfusion values (CBF, CBV, MTT, and CTH) between groups (patients, young healthy subjects and old healthy subjects) were performed using linear regression analysis controlling for age. p<0.05 was considered statistically significant after adjusting for multiple comparisons.
RESULTS
CBF derived from GE and SE DSC data decreased in the brain regions with elevated tau uptake (Figure 1). The regions of decreased CBF derived from the SE data revealed micro-vascular abnormalities and appeared as sub-areas within the region of decreased CBF derived from the GE data, which was dominated by both microscopic and macroscopic vessels.Regional increases in CTH were observed in close proximity to elevated tau uptake and further extended into the periventricular area (Figure 2), an area known to be vulnerable in MCI and AD patients.
Comparing the patients to older healthy subjects, significantly reduced CBF was seen in multiple brain regions, including the caudate, nucleus accumbens, cerebral white matter, cingulate gyrus, frontal lobe, occipitotemporal gyrus and temporal lobe. Abnormally long MTT and large CTH were also observed in regions such as insular cortex, cingulate gyrus, occipital lobe, and the hippocampus. No statistically significant difference was found on comparison of patients to young healthy subjects, likely due to the very small number of young healthy subjects.
DISCUSSION
Reduced CBF, prolonged MTT and large CTH were observed in MCI/AD patients as compared to healthy older subjects, consistent with prior work (2,4,6-8,18). Compromised blood brain barrier or tortuous capillaries may cause heterogeneous blood flow and inefficient oxygen extraction, which are reflected in the elevated MTT and CTH (8). These preliminary results are consistent with findings in AD mouse models that uncover surprising evidence of morphological and functional alterations in the capillaries in areas of tau over-expression (6). Future work will focus on recruiting more age-matched healthy subjects and AD patients to increase statistical power.CONCLUSION
The ability to acquire both GE and SE DSC data using the recently developed SAGE DSC-MRI sequence has enabled us to probe and potentially distinguish micro- and macro-vascular contributions to perfusion with only one injection of a standard dose of Gd. Our ongoing efforts to combine the GE and SE DSC data in Vessel Architectural Imaging (VAI) analyses (24-26) promise to provide estimates of vessel caliber and degree of vessel branching (27).Acknowledgements
We appreciate the financial support from NIH R01EB20613, P30AG062421, K99AG061259, and R01NR010827.References
1. Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction-The disregarded partner of Alzheimer's disease. Alzheimers Dement. 2019;15(1):158-167.2. Sweeney MD, Kisler K, Montagne A, et al. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21(10):1318-1331.3. Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18(7):419-434.4. Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296-302.5. Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270-276.6. Iturria-Medina Y, Sotero RC, Toussaint PJ, et al. Alzheimer's Disease Neuroimaging I: Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934.7. Eskildsen SF, Gyldensted L, Nagenthiraja K, et al. Neurobiology of Aging 2017;50:107-118.8. Nielsen RB, Egefjord L, Angleys H, et al. Capillary dysfunction is associated with symptom severity and neurodegeneration in Alzheimer's disease. Alzheimer’s & Dementia 2017;13:1143-1153.9. Boxerman JL, Hamberg LM, Rosen BR, et al. MR contrast due to intravascular magnetic susceptibility perturbations. MRM. 1995;34(4):555-566.10. Fisel CR, Ackerman JL, Buxton RB, et al. MR contrast due to microscopically heterogeneous magnetic susceptibility: numerical simulations and applications to cerebral physiology. MRM. 1991;17(2):336-347.11. Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. MRM. 1994;31(1):9-21.12. Stadlbauer A, Zimmermann M, Heinz G, et al. Magnetic resonance imaging biomarkers for clinical routine assessment of microvascular architecture in glioma. JCBFM. 2017;37(2):632-643.13. Manhard MK, Bilgic B, Liao C, et al. Accelerated whole-brain perfusion imaging using a simultaneous multislice spin-echo and gradient-echo sequence with joint virtual coil reconstruction. MRM. 2019;82(3):973-983.14. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-19.15. Center for Functionally Integrative Neuroscience, Aarhus University, Denmark Penguin, http://www.cfin.au.dk/penguin 16. Mouridsen K, Christensen S, Gyldensted L, et al. MRM. 2006; 55:524–531.17. Mouridsen K, Friston K, Hjort N, et al. NeuroImage. 2006; 33:570–579.18. Jespersen SN and Østergaard L. JCBFM. 2012; 32:264–277.19. Mouridsen K, Hansen B, Østergaard L, et al. JCBFM. 2014; 34:1511–1521.20. Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-80.21. Iglesias, JE, Augustinack, JC, Nguyen, K, et al. Neuroimage. 2015;115:117-137.22. Saygin ZM, Kliemann D, Iglesias JE, et al. Neuroimage. 2017;155:370-382.23. Iglesias JE, Van Leemput K, Augustinack J, et al. Neuroimage. 2016;141:542-555.24. Emblem KE, Mouridsen K, Bjornerud A, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med. 2013;19(9):1178-1183.25. Stadlbauer A, Zimmermann M, Heinz G, et al. Magnetic resonance imaging biomarkers for clinical routine assessment of microvascular architecture in glioma. JCBFM. 2017;37(2):632-643.26. Xu C, Kiselev VG, Moller HE, et al. Dynamic hysteresis between gradient echo and spin echo attenuations in dynamic susceptibility contrast imaging. MRM. 2013;69(4):981-991.27. Digernes I, Bjornerud A, Vatnehol SAS, et al. A theoretical framework for determining cerebral vascular function and heterogeneity from dynamic susceptibility contrast MRI. JCBFM. 2017;37(6):2237-2248.Figures
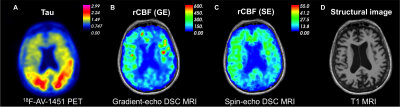
Figure 1: Preliminary
results demonstrating (A) prominent neocortical tau uptake in the bilateral
parietal lobes in a 76-year-old female with Alzheimer’s disease. Cerebral blood
flow (CBF) maps derived from (B) gradient echo and (C) spin echo DSC MRI show
regional decreases in relative CBF (rCBF) corresponding to areas of high tau
uptake. The rCBF map derived from gradient echo DSC (B) reflects blood flow in
large and small vessels, while the spin echo rCBF (C) map is predominantly
sensitive to microscopic vessels.
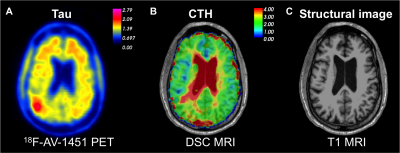
Figure 2: Regional increase in
capillary transit time heterogeneity (CTH) in the right parietal lobe (B), in
close proximity of the area with elevated tau uptake (A) in a 70-year-old man
with MCI.