1458
White Matter Integrity and Cortical Structure – Dependence on Apolipoprotein E Homozygous ε4 Allele Status
Harald Kugel1, Jonathan Repple2, Janik Goltermann2, Ronny Redlich2, Katharina Dohm2, Claas Kaehler2,3, Dominik Grotegerd2, Katharina Foerster2, Susanne Meinert2, Verena Enneking2, Tim Hahn2, Andreas Jansen4, Axel Krug4, Igor Nenadic4, Simon Schmitt4, Frederike Stein4, Tina Meller4, Dilara Yueksel4, Elena Fischer4, Marcella Rietschel5, Stephanie Witt5, Andreas J Forstner6,7, Markus Noethen6, Andreas Johnen8, Tilo Kircher4, Bernhard T Baune2, Udo Dannlowski2, and Nils Opel2
1Institute of Clinical Radiology, University of Muenster, Muenster, Germany, 2Department of Psychiatry, University of Muenster, Muenster, Germany, 3Department of Mathematics and Computer Science, University of Muenster, Muenster, Germany, 4Department of Psychiatry, University of Marburg, Marburg, Germany, 5Department of Genetic Epidemiology in Psychiatry, University of Heidelberg, Mannheim, Germany, 6Institute of Human Genetics, University of Bonn, Bonn, Germany, 7Centre for Human Genetics, University of Marburg, Marburg, Germany, 8Department of Neurology, University of Muenster, Muenster, Germany
1Institute of Clinical Radiology, University of Muenster, Muenster, Germany, 2Department of Psychiatry, University of Muenster, Muenster, Germany, 3Department of Mathematics and Computer Science, University of Muenster, Muenster, Germany, 4Department of Psychiatry, University of Marburg, Marburg, Germany, 5Department of Genetic Epidemiology in Psychiatry, University of Heidelberg, Mannheim, Germany, 6Institute of Human Genetics, University of Bonn, Bonn, Germany, 7Centre for Human Genetics, University of Marburg, Marburg, Germany, 8Department of Neurology, University of Muenster, Muenster, Germany
Synopsis
Apoliprotein E (APOE) genotype status, a predictor of Alzheimer's disease, has been associated with brain structural alterations. Supplementing previous research that primarily focused on hippocampal morphometry, we found a widespread effect of APOE allele status on cortical surface area and white matter microstructure. There is a significant cortical surface area decrease in 57 out of 68 cortical brain regions in APOE ε4 homozygous carriers. Furthermore, APOE ε4 homozygous carriers showed significant and widespread reductions in fractional anisotropy in corpus callosum, right internal and external capsule, left corona radiata and right fornix.
Introduction
Apolipoprotein E (APOE) genotype status is the strongest single gene predictor of Alzheimers disease (AD).1 APOE genotype has frequently been associated with AD related brain structural alterations before the onset of dementia.2,3 While previous research has primarily focused on hippocampal morphometry related to APOE, more recent findings have questioned the specificity of the hippocampus and instead suggested global effects of APOE on gray and white matter.4,5 Yet, current knowledge on effects of APOE on cortical brain structure and white matter microstructure is limited up to now. With the present study we aimed to investigate associations between APOE allele status and cortical gray matter structure as well as white matter microstructure.Methods
To investigate the effect of APOE genotype status, APOE carriers homozygous for the ε4 allele (ε4/ε4) were contrasted with subjects without an ε4 allele (genotypes ε2/ε2, ε2/ε3 or ε3/ε3). Morphometry analyses included a) FreeSurfer based cortical segmentations of thickness and surface area measures and b) tract based spatial statistics (TBSS) of DTI measures. Participants were selected from a large cohort (N = 1141) with complete MRI and neuropsychological data,6 which includes subjects with Major Depression (MDD) and healthy controls (HC). The study sample was selected based on the genotype of the APOE gene and consisted of 31 homozygous ε4 carriers and 31 non-ε4 carriers, matched for age and gender, 24 MDD patients and 38 HCs, mean age 34.5±13.5 years. MR data were acquired on two 3T systems (Tim Trio and Prisma, Siemens), using a 3D MP-RAGE sequence for gray matter analysis (isotropic voxels 1 mm edge length, TR/TE/TI 2130 ms/2,3 ms)/900 ms) and DTI imaging (isotropic voxels 2.5 mm edge length, TR/TE 7300 ms/90 ms, b = 0 and 1000 s/mm2, 30 directions) for Fractional Anisotropy (FA) analysis. Processing and analysis was performed with FSL5.0.10.7 Freesurfer (V 5.3) was used to obtain individual cortical surface area and thickness measures for 68 brain regions, using default parameters for cortical segmentation. Total cortical surface area and mean cortical thickness per hemisphere were used as global cortical structures measures, in addition to total intracranial volume (TIV). TBSS was performed with standard preprocessing.8,9 Genotyping was performed as described in a previous paper.10 Statistical analysis was done using SPSS (IBM Version 25). Both, for gray matter structure and FA, genotype group differences were established using general linear models (GLM) implemented in FSL.Results
APOE and cortical gray matter structure: In multivariate GLM ε4 homozygotes showed significant reductions in multivariate measures of cortical thickness and surface area with a large effect size (F[5,49]=8.036, p<.001, η²=.451). Univariate follow-up analyses revealed that this multivariate effect was driven by significantly smaller surface area in left and right hemispheres respectively, and by significantly reduced TIV, whereas no genotype differences were found regarding mean cortical thickness in either hemisphere. False discovery rate (FDR) corrected significant regional cortical differences between genotype groups in favor of non-ε4 carriers were found in 57 of 68 regions regarding cortical surface area (smaller surface area in ε4 homozygotes). Regarding cortical thickness only 2 of 68 regions were significant with larger thickness in ε4 homozygotes, in the direction contrary to our previous hypothesis. Genotype effects on regional surface area were widespread over the whole brain with strongest differences in the frontal and superior temporal regions (Figure 1).APOE and white matter integrity: We found significantly reduced FA in homozygous ε4 carriers (pFWE <0.05) in several clusters including the body of the corpus callosum, right internal and external capsule, left corona radiata and right fornix (see Figure 2). We did not find clusters with significantly higher FA in the homozygous ε4 carriers. Exploratory analyses did not reveal any significant group*genotype (p= .89), genotype*age (p= .42) or group*age (p= .64) interactions.
Discussion
We found a detrimental impact of homozygous APOE ε4 genotype on white matter integrity and widespread cortical surface area with strongest effects in frontal and temporal regions. Reduced FA is found in two major clusters, which include both hemispheres as well as several major white matter tracts like corpus callosum, internal and external capsule and corona radiata. This is line with several DTI studies in healthy APOE heterozygous ε4 carriers and supports a concept of a global adverse effect of ε4 genotype on widespread white matter pathways, rather than specific, localized effects. Interestingly, while effects for surface area were extensive, we could not detect any hypothesized effects on cortical thickness, which suggests a more specific association of APOE and surface area. As shown in previous work,10 young healthy homozygous ε4 carriers perform less on verbal working memory tasks. This effect could be mediated by our findings of widespread grey and white matter alterations in this group, as both grey matter volume as well as white matter integrity has been associated with performance in working memory tasks.11Conclusions
The present findings confirm a global rather than regionally specific effect of APOE ε4 allele status on brain structure. APOE ε4 homozygous allele carriers show widespread impairments both in white matter integrity and cortical grey matter surface in young adults, which could be a risk factor for the future development of Mild Cognitive Impairment (MCI) and AD.Acknowledgements
This work was funded by the German Research Foundation (DFG, grant FOR2107 DA1151/5-1 and DA1151/5-2 to UD).References
1. Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013; 45(12):1452-1458. 2. Lemaître H, Crivello F, Dufouil C, et al. No ε4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. Neuroimage. 2005; 24:1205–1213. 3. Wishart HA, Saykin AJ, McAllister TW, et al. Regional brain atrophy in cognitively intact adults with a single APOE epsilon 4 allele. Neurology. 2006; 67:1221–1224. 4. Liu Y, Paajanen T, Westman E, et al. Effect of APOE ε4 allele on cortical thicknesses and volumes: the AddNeuroMed study. J Alzheimers Dis. 2010; 21(3):947-966. 5. Filippini N, Rao A, Wetten S, et al. Anatomically-distinct genetic associations of APOE epsilon4 allele load with regional cortical atrophy in Alzheimer's disease. Neuroimage. 2009; 44(3):724-728. 6. Kircher T, Wöhr M, Nenadic I, et al. Neurobiology of the major psychoses. A translational perspective on brainstructure and function: the FOR2107 consortium. Eur Arch Psychiatry Clin Neurosci 2018. doi: 10.1007/s00406-018-0943-x. [Epub ahead of print]. 7. FMRIB Software Library. https://fsl.fmrib.ox.ac.uk/fsl/fslwiki – Version history and release notes. Accessed October 30, 2019. 8. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006; 31(4):1487-1505. 9. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009; 44(1):83-98. 10. Goltermann J, Redlich R, Dohm K, et al. Apolipoprotein E Homozygous +4 Allele Status: A Deteriorating Effect on Visuospatial Working Memory and Global Brain Structure. Front Neurology. 2019; 10:552. 11. Repple J, Karliczek G, Meinert S, et al. Variation of HbA1c affects cognition and white matter microstructure in healthy, young adults. Mol Psychiatry. 2019. doi: 10.1038/s41380-019-0504-3. [Epub ahead of print].Figures
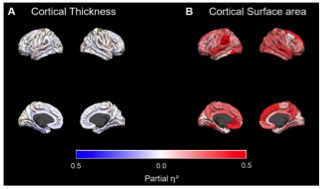
Figure 1: Effect sizes of genotype group differences in thickness and surface area for each cortical region. Red color indicates larger thickness or surface area for non-ε4 carriers compared to ε4 homozygotes. Blue color indicates the reverse contrast. Effect sizes for all regions are presented including non-significant differences
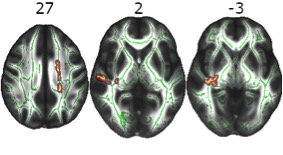
Figure 2: Reduced Fractional Anisotropy in homozygous ε4 carriers. Axial slices with corresponding z-axis values (MNI-coordinates). Red-yellow areas represent voxels (using FSL’s „fill“ command for better visualization), where a significant reduction in fractional anisotropy in homozygous ε4 carriers was detected (pFWE< .05).