1455
Mean diffusivity in white matter correlates negatively with episodic memory performance in aging, non-demented adults with Down Syndrome1University of Wisconsin-Madison, Madison, WI, United States, 2University of Pittsburgh Medical Center, Pittsburgh, PA, United States, 3Columbia University, New York, NY, United States, 4University of Cambridge, Cambridge, United Kingdom
Synopsis
People with Down Syndrome (DS) are at an increased risk of developing Alzheimer’s Disease (AD), due to an overproduction of β-amyloid resulting from triplication of the amyloid precursor protein (APP) gene located on chromosome 21. The effect of white matter degradation on early AD-related cognitive decline in the DS population is not known. In this work, we present results from a study of non-demented adults with DS showing correlations between increased mean diffusivity (MD), derived using diffusion tensor imaging (DTI), within several white matter regions of interest and poorer performance on measures of episodic memory.
Introduction
Nearly all persons with Down Syndrome (DS) have indications of Alzheimer’s Disease (AD) pathology by age 50 years.1 This is purported to be due to the increased accumulation of β-amyloid resulting from the over expression of amyloid precursor protein (APP) gene, which is located on chromosome 21.2 β-Amyloid has been linked with decreased gray and white matter health in both cognitively healthy and demented populations.3 Given the early deposition of β-amyloid in DS, it is important to assess its association with white matter (WM) health and cognitive performance. Episodic memory has been observed as one of the first cognitive domains to decline in aging DS subjects.4 In this study, we extracted mean FA and MD values within subcortical white matter regions of interest (ROIs) and performed partial correlation analysis with a composite score of episodic memory assessments. Data were collected from a sample of non-demented adults with DS.Methods
Forty-seven (n=47) adults with DS (mean age=39.8 +/- 7.0 years) were imaged at 3T at either the University of Wisconsin-Madison (n=28) or the University of Pittsburgh (n=19) and completed a cognitive battery. The DTI protocol consisted of a single shell, b=1000s/mm2, with 48 encoding directions and 1.875mm x 1.875mm x 2.500mm resolution. Data were processed using an in-house processing pipeline utilizing DIPY5, FSL6, and Mrtrix7. Processing included corrections for Rician noise8, Gibbs ringing9, and eddy-currents10,11. The tensor model was then fitted using the DiPy toolkit using weighted least squares. Images were spatially normalized to the FMRIB 1mm FA12 template using advanced normalization tools (ANTs)13 and several subcortical white matter ROIs were extracted using the JHU White Matter Atlas14. The mean values of fractional anisotropy (FA) and mean diffusivity (MD) in these regions were calculated. A composite score of episodic memory (EMCS) was calculated based on summing the z scores of: 1) Free and Cued Recall test15, a measure of verbal episodic memory and 2) Rivermead Behavioural Memory Test16 for Children Picture Recognition test, a measure of visual episodic memory. Three subjects were excluded due to incomplete cognitive data, bringing the total number of participants to forty-four (n=44). The association between episodic memory and DTI measures were analyzed using a partial correlation method in R and results were corrected for age, imaging site, and baseline intelligence. Baseline intelligence was measured using the Peabody Picture Vocabulary Test (PPVT).17Results
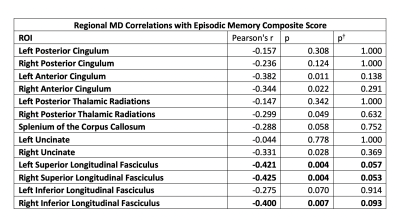
In Table 1, we observe several significant correlations between MD values within white matter regions and episodic memory performance. Regions of effect include the bilateral superior longitudinal fasciculi and anterior cingulum, as well as the right posterior thalamic radiations, inferior longitudinal fasciculus, and uncinate. No statistically significant correlations were observed for FA.Discussion
In this study, we were able to relate MD with episodic memory performance in non-demented adults with DS. Although, neuroimaging studies of DS are challenging, careful quality control and minor correction of the raw DWI data provided useable DTI measures. Careful non-linear registration methods were employed to ensure accurate image alignment and ROI extraction. Increases in MD have been reported in several imaging studies as correlated with poorer cognitive performance.18,19 Further, higher scores on the cognitive battery used in this study indicate greater cognitive ability. We observed several negative correlations between MD and episodic memory performance. Our results indicate a relation, independent of age and baseline intelligence, between performance on measures of episodic memory and measures of white matter microstructure. Our findings were observed in regions related to episodic memory, namely the thalamic radiations, cingulum, uncinate, and the superior and inferior longitudinal fasciculi; indications of white matter degeneration in these areas have been negatively correlated with episodic memory performance in normal aging in the general population.20 The studied population is relatively young and non-demented, and severely diminished cognitive performance would not be expected until much later. Further, cognitive impairments are an inherent aspect of DS. These effects likely resulted in lower correlation coefficients observed in this study. Future work will involve investigating the relation between DTI assessments of white matter, amyloid content within these ROIs using positron emission tomography (PET), and cognitive performance.Acknowledgements
We’d like to thank the participants and their families for their time and commitment to further discovery and understanding of the causes of AD.
NIH Funding R01AG031110, U54HD090256, U01AG051406
References
1,2Lao, P. J., Handen, B. L., Betthauser, T. J., Mihaila, I., Hartley, S. L., Cohen, A. D., Tudorascu, D. L., Bulova, P. D., Lopresti, B. J., Tumuluru, R. V., Murali, D., Mathis, C. A., Barnhart, T. E., Stone, C. K., Price, J. C., Devenny, D. A., Mailick, M. R., Klunk, W. E., Johnson, S. C., ... Christian, B. T. (2017). Longitudinal changes in amyloid positron emission tomography and volumetric magnetic resonance imaging in the nondemented Down syndrome population. Alzheimer's & dementia (Amsterdam, Netherlands), 9, 1-9. doi:10.1016/j.dadm.2017.05.001
3Zerbi, V., Kleinnijenhuis, M., Fang, X., Jansen, D., Veltien, A., Asten, J. V., … Heerschap, A. (2013). Gray and white matter degeneration revealed by diffusion in an Alzheimer mouse model. Neurobiology of Aging, 34(5), 1440–1450. doi: 10.1016/j.neurobiolaging.2012.11.017
4Hartley, S. L., Handen, B. L., Devenny, D., Mihaila, I., Hardison, R., Lao, P. J., … Christian, B. T. (2017). Cognitive decline and brain amyloid-β accumulation across 3 years in adults with Down syndrome. Neurobiology of aging, 58, 68–76. doi:10.1016/j.neurobiolaging.2017.05.019
5Garyfallidis, E., Brett, M., Amirbekian, B., Rokem, A., van der Walt, S., Descoteaux, M., … Dipy Contributors (2014). Dipy, a library for the analysis of diffusion MRI data. Frontiers in neuroinformatics, 8, 8. doi:10.3389/fninf.2014.00008
6,12M. Jenkinson, C.F. Beckmann, T.E. Behrens, M.W. Woolrich, S.M. Smith. FSL. NeuroImage, 62:782-90, 2012
7Tournier, J. D., Calamante, F., & Connelly, A. (2012). MRtrix: diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology, 22(1), 53-66.
8Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394-406.
9Kellner, E; Dhital, B; Kiselev, V.G & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine, 2016, 76, 1574–1581.
10Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-78.
11Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556-72
13Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A., & Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54(3), 2033-2044.
14Oishi, K., Zilles, K., Amunts, K., Faria, A., Jiang, H., Li, X., Akhter, K., Hua, K., Woods, R., Toga, A. W., Pike, G. B., Rosa-Neto, P., Evans, A., Zhang, J., Huang, H., Miller, M. I., van Zijl, P. C., Mazziotta, J., ... Mori, S. (2008). Human brain white matter atlas: identification and assignment of common anatomical structures in superficial whitematter. NeuroImage, 43(3), 447-57
15Buschke H. Cued recall in amnesia. Journal of Clinical and Experimental Neuropsychology. 1984;6:433–440.
16Wilson B, Ivani-Chalian C, Aldrich F. Rivermead behavioral memory test for children. Bury St Edmunds, U.K.: Thames Valley Test Co.; 1991.
17Dunn, Lloyd M.Dunn, Douglas M. (2007) PPVT-4 :Peabody picture vocabulary test Minneapolis, MN. : Pearson Assessments.
18Mayo, C. D., Mazerolle, E. L., Ritchie, L., Fisk, J. D., Gawryluk, J. R., Alzheimer's Disease Neuroimaging Initiative (2016). Longitudinal changes in microstructural white matter metrics in Alzheimer's disease. NeuroImage. Clinical, 13, 330-338. doi:10.1016/j.nicl.2016.12.012
19Kantarci, K., Murray, M. E., Schwarz, C. G., Reid, R. I., Przybelski, S. A., Lesnick, T., … Dickson, D. W. (2017). White-matter integrity on DTI and the pathologic staging of Alzheimer's disease. Neurobiology of aging, 56, 172–179. doi:10.1016/j.neurobiolaging.2017.04.024
20Lockhart, S. N., Mayda, A. B., Roach, A. E., Fletcher, E., Carmichael, O., Maillard, P., … Decarli, C. (2012). Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Frontiers