1448
Assessing the impact of age-related locus coeruleus degeneration on cognitive decline1Center for Advanced Neuroimaging, University of California Riverside, Riverside, CA, United States, 2Bioengineering, University of California Riverside, Riverside, CA, United States, 3Psychology, University of California Riverside, Riverside, CA, United States, 4Neurology, Emory University, Atlanta, GA, United States
Synopsis
Characterization of age-related alterations in microstructure of locus coeruleus and its projections will aid in the development of new biomarkers and may provide insight in the development of novel interventions to arrest progression of Alzheimer's disease or Parkinson's disease. Imaging locus coeruleus with diffusion-weighted images is difficult due to its small stature (locus coeruleus is 1.5 mm in diameter and 15 mm long) and location in the brain stem. In this abstract, we utilize a high resolution diffusion-weighted protocol to examine age-related microstructural changes in locus coeruleus and examine its effect on age-related cognitive decline.
Introduction
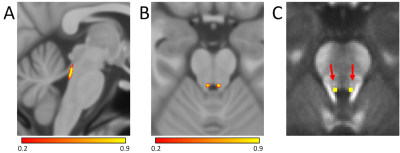
Locus coeruleus (LC) is a small catecholaminergic structure located along the dorsal edge of the brainstem just anterior to the 4th ventricle (see Figure 1) and is approximately 1.6 mm in diameter and 15 mm long. Its projections innervate many areas of the brain including regions involved in learning and memory, such as the neocortex, thalamus, and hippocampus1. Specifically, the dorsal portions of thalamus are involved in working memory2 and dorsal thalamic nuclei receive a large output from LC3 with retrograde tracer studies in macaques finding fibers arising from the rostral portions of LC enter the dorsomedial area of the central tegmental tract and eventually branch to the thalamus4,5.Norepinephrine protects neurons from oxidative stress and reduces inflammation6,7 and LC is the primary source of norepinephrine to much of the brain1. This protective effect is the basis of the LC-reserve hypothesis by which activation of LC and the release of norepinephrine may offer neuroprotection to neurons innervated by LC8. In particular, higher density of neurons in LC has been found to correlate with a reduction in cognitive decline9. Here, we explore the relationship between retention of cognitive abilities and microstructure of LC and its projection to the thalamus using a high resolution DTI protocol.
Methods
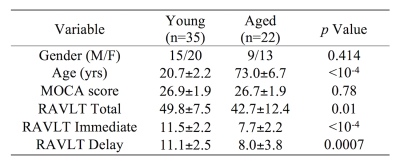
A cohort consisting of 57 subjects (22 aged and 35 young subjects) were scanned in this study. Montreal cognitive assessment scoring (MOCA) and Rey Auditory Verbal Learning Test (RAVLT) scores were collected on each subject to assess cognitive performance. Relationships with MRI measures were found individually for each group using Spearman’s rank correlations. All subjects gave written, informed consent and demographic data is summarized in Table 1.Imaging data were acquired on a 3 T MRI scanner (Prisma, Siemens Healthineers, Malvern, PA) using a 32-channel receive only coil. Images from a MP-RAGE sequence (echo time (TE)/repetition time (TR)/inversion time=3.02/2600/800 ms, flip angle=8°, voxel size=0.8×0.8×0.8 mm3) were used for registration from subject space to common space.
High-resolution diffusion MRI data were collected with a single-shot spin-echo, echo planar imaging sequence. A dual spin-echo technique combined with monopolar gradients was used with the following parameters: TE/TR=97/3292 ms, voxel size=0.95×0.95×1 mm3, 64 slices with no gap, multiband factor=2. Diffusion-weighting gradients were applied in 30 directions with two b values of 500 s/mm2 and 2000 s/mm2. A set of 32 b=0 images with phase-encoding directions of opposite polarity were acquired to correct for susceptibility distortion.
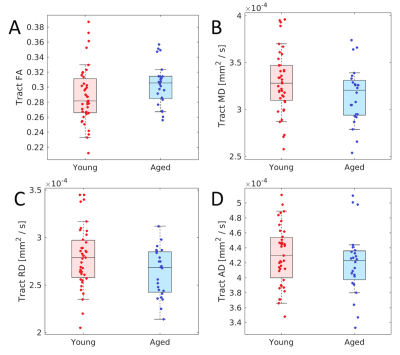
DTI data were corrected for eddy current and susceptibility distortions using eddy in FSL. Principal diffusion directions and crossing fibers were estimated using dtifit and bedpostX in FSL. Finally, probabilistic tractography, as implemented in FSL, was used to track the fiber tracts originating from a standardized locus coeruleus atlas10 to a thalamus atlas from the Harvard-Oxford atlas. Fractional anisotropy (FA), mean diffusivity (MD), and radial diffusivity (RD) were measured in LC and the resulting LC-thalamus tracts.
Results
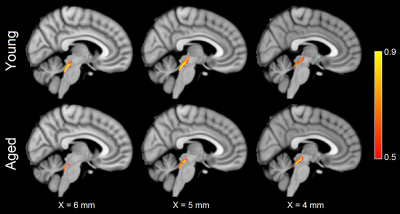
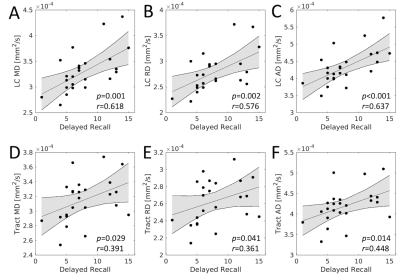
For each group, tracts leaving the LC seed mask were contained within a region anatomically consistent with the central tegmental tract and projecting to the dorsal portion of the thalamus (see Figure 2). These projections agree with tracer studies in the macaque4,5. Relative to young, an increase in FA (p=0.04) and decrease in MD (p=0.04) was observed in the tract going from LC to thalamus in the aged cohort. These changes were driven by reductions in radial diffusivity within the tract in the aged cohort (p=0.016). A trend toward decreased AD (p=0.16) was seen in the aged cohort. These results are summarized in Figure 3.As shown in Figure 4, significant correlations were seen between diffusion metrics in the LC-thalamus tract and RAVLT delayed recall in the aged group (mean tract MD: r=0.391,p=0.029; mean tract RD: r=0.361,p=0.041; mean tract AD: r=0.448,p=0.014) but not in the young group (mean tract MD: r=0.148,p=0.184; mean tract RD: r=-0.166,p=0.156; mean tract AD: r=-0.073,p=0.330).
The effect of LC microstructure on memory was assessed by correlating LC diffusion indices (MD, RD, and AD) with memory performance (RAVLT delayed recall). In the aged group, mean LC diffusion metrics positively correlate with RAVLT delayed recall, indicating that as memory performance increases, mean LC MD (r=0.618,p=0.001), mean LC RD (r=0.576,p=0.002), and mean LC AD (r=0.637,p=4.1x10-4) also increase. Interestingly, no correlation between any LC diffusion measures and RAVLT delayed recall score was observed in the young group (mean LC MD: r=0.074,p=0.487; mean LC RD: r=0.006,p=0.346; mean LC AD: r=0.119,p=0.262).
Discussion
We found that lower performance on RAVLT delayed recall scores was correlated with reduced LC MD and RD (potentially reflecting reduced axon size), suggesting that LC microstructure integrity may be important in the retention of cognitive abilities during aging. Importantly, this result accords with both histology and an earlier imaging study, both of which found a link between LC contrast and the retention of cognitive reserve and the total number of LC cells9 or LC contrast11. A strong correlation was observed between AD and RAVLT delayed recall score in the LC-thalamus tract. Lower AD was correlated with worse memory performance in the aged group and may suggest that connectivity between LC and thalamus plays a role in the retention of cognitive abilities.Acknowledgements
This work was also supported by NIH-NIA grants R00 AG047334, R21 AG054804, and 1R34AG056639-01A1 as well as NIH-NINDS 1K23NS105944-01A1.References
[1] Aston-Jones and Cohen. Adaptive gain and the role of the locus coeruleus norepinephrine system in optimal performance. J Comp Neurol 493:99-110.
[2] Bolkan, et al. Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci 20:987-996.
[3] Samuels and Szabadi. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol 6:235-253.
[4] Tanaka et al. Histochemical mapping of catecholaminergic neurons and their ascending fiber pathways in the rhesus monkey brain. Brain Res Bull 9:255-270.
[5] Bowden et al. An autoradiographic, semistereotaxic mapping of major projections from locus coeruleus and adjacent nuclei in Macaca mulatta. Brain Res 145:257-276.
[6] Troadec et al. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J Neurochem 79:200-210.
[7] Feinstein et al. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem Int 41:357-365.
[8] Robertson. A noradrenergic theory of cognitive reserve: implications for Alzheimer's disease. Neurobiol Aging 34:298-308.
[9] Wilson et al. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 80:1202-1208.
[10] Chen, et al. Simultaneous imaging of locus coeruleus and substantia nigra with a quantitative neuromelanin MRI approach. Magn Reson Imaging. 32:1301-1306.
[11] Clewett et al. Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol Aging 37:117-126.
Figures