1446
Age-associated changes in cerebral blood flow-related measures using arterial spin labeling1Computer Science, State University of New York at Binghamton, Binghamton, NY, United States, 2Human Development, Cornell University, Ithaca, NY, United States
Synopsis
Disrupted CBF-related measures in clinical diseases has highlighted the importance of reliable baseline data from normal controls. We characterized CBF-related measures in normal aging by acquiring PCASL images in 11 young and 12 elderly subjects. We found that the elderly exhibited longer ATT in the frontal, parietal, temporal, and subcortical regions, reduced CBF in the prefrontal and parietal regions and increased CBF in the subcortical regions, and increased LFF in the superior frontal and paracentral region, compared to the young. This work sets the stage for a larger scale use of PCASL to investigate CBF-related alterations in neurological diseases.
Introduction
Sufficient cerebral blood flow (CBF) is crucial to brain neural function. CBF-related measures are key indicator of cerebral health. For instance, Alzheimer’s disease (AD), stroke, and hypertension are associated with disrupted CBF and related measures [1]. The spatial patterns of CBF-related changes in normal aging are needed to serve as control features for further investigation of neurological diseases [2]. Currently, the patterns are not fully elucidated and not very consistent. The aim of the study is to identify and characterize the changes in CBF, arterial transit time, and low frequency fluctuation that occur during normal aging. The arterial transit time (ATT) maps were derived using arterial spin labeling (ASL) technique with different post-labeling delays. CBF and low frequency fluctuation (LFF) maps were derived from time series of ASL images.Methods
The study was conducted using a GE 3T MR 750 scanner. All healthy subjects (11 young subjects: 24.5 4.16 years, 5 females; 12 elderly subjects: 68.00 3.07 years, 5 females) received T1-weighted MPRAGE images, a low-resolution transit time acquisition [3] with five post-labeling delays of 0.7 s, 1.3 s, 1.9 s, 2.5 s, 3.0 s, and a dynamic pseudo-continuous arterial spin labeling (PCASL) sequence [4] with 30 three-dimensional ASL images.ATT maps were derived voxel-by-voxel by applying a noniterative algorithm to the ASL data with five post-labeling delays [3]. CBF maps were calculated using a standard kinetic model without ATT correction [5, 6]. LFF maps were calculated using the standard deviation map (across the time) divided by global mean signal. The ATT, CBF, and LFF maps were normalized to a standard MNI template using the T1-weighted images as an intermediate, and smoothed with a 8×8×8 mm3 Gaussian kernel. The maps between the young and elderly groups were compared using SPM8 via two-sampled t tests with gender as a covariate. The maps were normalized by global mean values to reduce the cross-subject variations. The statistical maps were thresholded using a voxel-level p value of 0.001. A cluster-level p value of 0.05 was used to detect significant regions after correction of multiple comparisons.
Results & Discussion
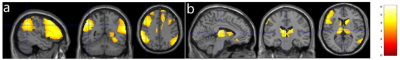
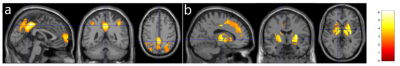
ATT values in the elderly subjects were significantly longer in the frontal, parietal, and temporal (Fig. 1a), and subcortical areas (Fig. 1b) than those in the young subjects, which is consistent with our earlier results [7]. CBF values in the elderly subjects were significantly reduced in the prefrontal and parietal areas (Fig. 2a) but significantly increased in the subcortical areas (Fig. 2b). The regions with reduced CBF in the elderly are in good agreement with previous PET studies [8]. The increased CBF values in the subcortical regions of the elderly agrees well with some literature [9, 10] due to inconsistent findings from the past studies. These increased CBF values can be potentially caused by our global normalization. However, we found that the increased CBF values in the elderly remained significant even without global normalization. Further investigation is planned for the effect of ATT correction on the elderly CBF. LFF values in the elderly were found significantly increased in the superior frontal and paracentral area (Fig. 3). This area seemed to be a potential targeted area as the LFF in the area showed a trend to follow the disease progression of AD [11].Conclusion
This study demonstrated that ASL can sensitively detect the normal aging effects using a small number of subjects. This work sets the stage for a larger scale use of PCASL to investigate CBF-related alterations in cerebrovascular disease and AD.Acknowledgements
No acknowledgement found.References
1. Girouard, H. and C. Iadecola, Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985), 2006. 100(1): p. 328-35.
2. Calne, D.B., A. Eisen, and G. Meneilly, Normal aging of the nervous system. Ann Neurol, 1991. 30(2): p. 206-7.
3. Dai, W., et al., Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med 2012. 67(5): p. 1252-65.
4. Dai, W., et al., Quantifying fluctuations of resting state networks using arterial spin labeling perfusion MRI. J Cereb Blood Flow Metab, 2016. 36(3): p. 463-73.
5. Alsop, D.C. and J.A. Detre, Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of Human cerebral blood flow. J Cereb Blood Flow Metab, 1996. 16: p. 1236-49.
6. Buxton, R.B., et al., A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med, 1998. 40: p. 383-96.
7. Dai, W., et al., Effects of arterial transit delay on cerebral blood flow quantification using arterial spin labeling in an elderly cohort. J Magn Reson Imaging, 2017. 45(2): p. 472-481.
8. Chen, J.J., H.D. Rosas, and D.H. Salat, Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage, 2011. 55(2): p. 468-78.
9. Lee, C., et al., Imaging cerebral blood flow in the cognitively normal aging brain with arterial spin labeling: implications for imaging of neurodegenerative disease. J Neuroimaging, 2009. 19(4): p. 344-52.
10. Pagani, M., et al., Regional cerebral blood flow as assessed by principal component analysis and (99m)Tc-HMPAO SPET in healthy subjects at rest: normal distribution and effect of age and gender. Eur J Nucl Med Mol Imaging, 2002. 29(1): p. 67-75.
11. Yang, L., et al., Gradual Disturbances of the Amplitude of Low-Frequency Fluctuations (ALFF) and Fractional ALFF in Alzheimer Spectrum. Front Neurosci, 2018. 12: p. 975.
Figures