1444
Brain aging and sexual dimorphism in myelination investigated using magnetic resonance myelin water fraction imaging1NIA, NIH, Baltimore, MD, United States
Synopsis
Changes
in brain myelination with age and sex have been the subject of intense MRI study.
However, most studies have used nonspecific methods to probe myelin, including DTI
and relaxation times. We investigated age- and sex-related differences in brain
myelin water fraction (MWF), a surrogate of myelin content, in a large cohort
of unimpaired participants, spanning a wide age range. We observed a quadratic relationship
between MWF and age in all brain regions investigated, suggesting that
myelination continues until middle age followed by a decrease through older age. Sexual dimorphism in this process was
observed in several brain regions.
PURPOSE
To investigate differences in myelin content in the brain with normative aging and sex. Our main goals are to characterize the regional association of myelination with age and sex in specific brain regions, to provide reference values for MWF in the adult brain, and to develop further insights into regional brain maturation and aging in a large life-span sample of healthy adults.METHODS
Subjects and MRI:The study cohort consisted of 106 subjects (55.1±21.3 years) of 48 women (53.4±20 years) and 58 men (56.7±22.5 years) spanning the age range between 22 and 94 years. Participants underwent a mini-mental state exam (MMSE men = 28.5±1.5; women = 29±1.4). Age and MMSE were not statistically different between men and women. For each participant, a whole brain MWF map was derived using BMC-mcDESPOT (1-3). For this, ten 3D spoiled-gradient-recalled-echo (SPGR) images acquired with flip angles (FAs) of [2 4 6 8 10 12 14 16 18 20]°, echo time of (TE) 1.37 ms and repetition time (TR) of 5 ms, and ten 3D balanced steady-state free-precession images acquired with FAs of [2 7 11 16 24 32 40 60]°, TE of 2.8 ms, TR of 5.8 ms, and radiofrequency excitation pulse phase increments of 0o or 180o to account for off-resonance effects (4). All images were acquired with a voxel size of 1.6 mm × 1.6 mm × 1.6 mm. Further, we used the DAM to correct for B1 inhomogeneity (5). The DAM protocol consists of two fast spin-echo images acquired with FAs of 45° and 90°, TE of 102 ms, TR of 3000 ms, and acquisition voxel size of 2.6 mm × 2.6 mm × 4 mm. All images were obtained with a field-of-view of 240 mm × 208 mm × 150 mm and reconstructed on the scanner to a voxel size of 1 mm × 1 mm × 1 mm.
Image processing and statistical analysis:
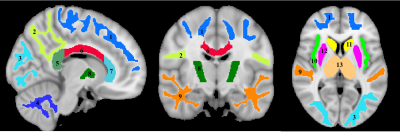
For each participant, a whole-brain MWF map was generated using the BMC-mcDESPOT analysis (1-3). Further, the averaged SPGR image over FAs was nonlinearly registered to the MNI space and the computed transformation matrix was then applied to the corresponding MWF map; this analysis was performed using the FSL software (6). Fourteen regions of interest (ROIs) were defined encompassing white matter (WM) and deep gray matter (GM) regions of the brain (Figure 1). Ten WM ROIs were derived from the MNI structural atlas, while the four deep GM regions were derived from the JHU atlas (6). Finally, For each ROI, the effects of age and sex on myelination were investigated using linear regression with the mean MWF within the ROI as the dependent variable and sex, age, and age2 as independent variables (7). Interactions between sex and age as well as sex and age2 were dropped if found not significant.
RESULTS & DISCUSSION
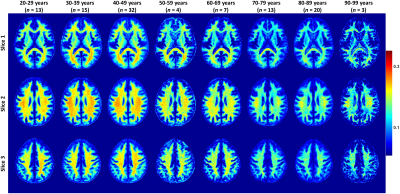
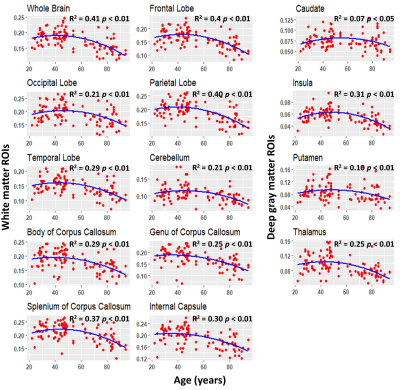
Figure 2 shows the average MWF maps by age decade over the adult lifespan. In agreement with Arshad’s and colleagues recent study (7), visual inspection and quantitative ROI analysis (Figs. 2-3) show increases in MWF values from early adulthood until middle age, followed by reduced MWF in several brain regions, suggesting progressive myelination followed by reductions in myelin. Different regions exhibit different pattern associations between MWF and age, with the occipital regions myelinating early and demyelinating late as compared to more anterior regions. This is in line with the retrogenesis (first in-last out) hypothesis, which suggests that anterior brain regions develop full myelination more slowly and then demyelinate more rapidly in response to aging and neurodegeneration, while posterior brain regions, which myelinate more rapidly, are also better preserved during these processes (8-10). Finally, the more posterior brain regions, especially the occipital and parietal lobes, exhibited greater MWF values as compared to the more anterior brain regions, while the lowest MWF values were found in the deep GM regions, as expected.A quadratic dependence on age, age2, was significant in all brain regions except in the genu of the corpus callosum. Further, all ROIs exhibited non-significant interactions between age2 and sex. However, the main effect of sex was significant in the parietal lobes, the body of corpus callosum, and the splenium. In these brain regions, women had 8-10% higher myelin content than men. Interaction between age and sex was also significant in the whole brain, the temporal lobes, and the body of corpus callosum. In these regions, women showed a non-significant trend to greater reduction in MWF with age as compared to men. These trends indicate potential lines of investigation in larger cohorts. Indeed, the sex differences we observed in myelin are consistent with previous demonstrations that proliferation of oligodendrocytes and myelin proteins are regulated differently in males and females (11-13). A recent study suggests that sex steroids may influence this differential regulation, possibly contributing to sex differences in myelin maintenance and repair (14).
CONCLUSIONS
We showed that myelin content follows an inverted-U shaped association with normal aging; this indicates brain maturation with respect to myelination until middle age followed by decreased myelination with advancing age. We also found significant sex differences in a several brain regions, with women exhibiting higher myelin content than men.Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.References
1. Bouhrara M, Spencer RG. Incorporation of nonzero echo times in the SPGR and bSSFP signal models used in mcDESPOT. Magnetic resonance in medicine. 2015;74(5):1227-35.
2. Bouhrara M, Spencer RG. Improved determination of the myelin water fraction in human brain using magnetic resonance imaging through Bayesian analysis of mcDESPOT. NeuroImage. 2016;127:456-71.
3. Bouhrara M, Spencer RG. Rapid simultaneous high-resolution mapping of myelin water fraction and relaxation times in human brain using BMC-mcDESPOT. NeuroImage. 2017;147:800-11.
4. Deoni SC. Correction of main and transmit magnetic field (B0 and B1) inhomogeneity effects in multicomponent-driven equilibrium single-pulse observation of T1 and T2. Magnetic resonance in medicine. 2011;65(4):1021-35.
5. Stollberger R, Wach P. Imaging of the active B1 field in vivo. Magnetic resonance in medicine. 1996;35(2):246-51.
6. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782-90.
7. Arshad M, Stanley JA, Raz N. Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. NeuroImage. 2016;143:26-39.
8. Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiology of aging. 2010;31(9):1554-62.
9. Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, et al. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer's disease supports retrogenesis. NeuroImage. 2009;45(1):10-6.
10. Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, et al. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiology of aging. 2012;33(8):1699-715.
11. Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(5):1439-47.
12. Yang S, Li C, Zhang W, Wang W, Tang Y. Sex differences in the white matter and myelinated nerve fibers of Long-Evans rats. Brain Research. 2008;1216:16-23.
13. Greer JM, Csurhes PA, Pender MP, McCombe PA. Effect of gender on T-cell proliferative responses to myelin proteolipid protein antigens in patients with multiple sclerosis and controls. Journal of Autoimmunity. 2004;22(4):345-52.
14. Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte Progenitor Proliferation and Maturation Is Differentially Regulated by Male and Female Sex Steroid Hormones. Developmental Neuroscience. 2004;26(2-4):245-54.
Figures