1434
Association of Myelination in Internal Capsule with Iron Deposition in the Basal Ganglia in Macaque monkeys1Institute of Neuroscience, CAS Center for Excellence in Brain Science and Intelligence Technology, State Key Laboratory of Neuroscience, CAS Key Laboratory of Primate Neurobiology, Chinese Academy of Sciences, Shanghai, China, 2Center for Brain Imaging Science and Technology, Key Laboratory for Biomedical Engineering of Ministry of Education, College of Biomedical Engineering and Instrumental Science, Zhejiang University, Hangzhou, China, 3Department of Radiology, Fujian Provincial Maternity and Children’s Hospital of Fujian Medical University, Fuzhou, China, 4Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, China, 5Department of Imaging Sciences, University of Rochester, Rochester, NY, United States
Synopsis
A tight association between iron and myelin content has been demonstrated in the local brain regions. However, it remains unknown whether such relationship exists between adjacent brain regions. Using 8 young and 8 older macaque monkeys, we integrated MRI-based QSM and MWF imaging to examine the relation between iron deposition in components of BG and the myelin content of BG-connecting IC. Our results reveal that the iron of BG is positively correlated with the myelin of IC at different aging stages, and demonstrate that iron in BG also affects the myelin content of the anatomically distinguished yet connected WM structures.
INTRODUCTION
Both myelin and iron play important roles in maintaining normal brain functional and metabolic activities on daily basis. Aggregating evidence has shown that myelination in the white matter (WM) continues into adulthood, and myelin degeneration occurs in old age 1-3, throughout which iron is essential for myelin synthesis and maintenance 4. It has been recognized high correspondence between iron and myelin contents in most gray matter (GM) regions whereas generally low correspondence in WM, thalamus (TH) and basal ganglia (BG) regions 5. However, the relationship between brain iron and myelin content in the BG and its anatomically connecting fiber tracts such as internal capsule (IC) remains essentially unknown. Here we aimed to measure the iron content of the BG areas including caudate, globus pallidus (GP) and putamen in the macaque brain by using the Quantitative Susceptibility Mapping (QSM) technique. Meanwhile by using Myelin Water Fraction (MWF) imaging, we measured the myelin content of the BG-connecting fiber tracts IC including ALIC and PLIC. Myelin content of four major WM structures including the optic tracts, the genu, body and splenium of corpus callosum that are anatomically unconnected with the BG was also measured as controls.METHODS
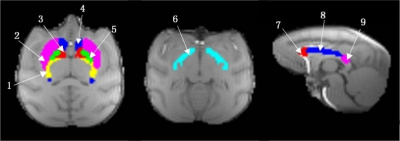
Sixteen healthy adult macaques (8 young , mean age 7.7 years, range 6.7-8.7 years; 8 older , mean age 24.7 years, range 23.4-26 years) were prepared similarly to our previous work 6-8 and scanned on a 3T Siemens Trio scanner with AC88 gradient-insert. A customized 3D multi-echo GRE sequence 9 was used for data acquisition: TR 60ms, 32 echoes, TE of 1st echo 2.4ms, echo spacing 1.42ms, matrix size 128×128×52, 1mm isotropic voxel size, flip angle 25 deg, 6 averages. The odd echoes acquired from the mGRE sequence were used for calculation of QSMs with STISuite v3.0 toolbox (https://people.eecs.berkeley.edu/~chunlei.liu/software.html). The MWF maps were calculated from the all 32 mGRE echoes, and the postprocessing was based on the method proposed by incorporating local tissue susceptibility to acquire high resolution myelin water imaging 9. The masks of 5 WM ROIs (GCC, BCC, SCC, IC, optic tract) and 3 BG ROIs (CN, GP and putamen) were derived from INIA19 atlas 10. IC was manually divided into two parts (ALIC, PLIC) using a line at the inflection point 11, 12. MWF or QSM values were then acquired from all ROIs in the native space of each macaque (shown in Figure 1 as an example). Statistical analysis was performed with SPSS Version 20 (IBM Inc., Armonk, NY, USA).RESULTS
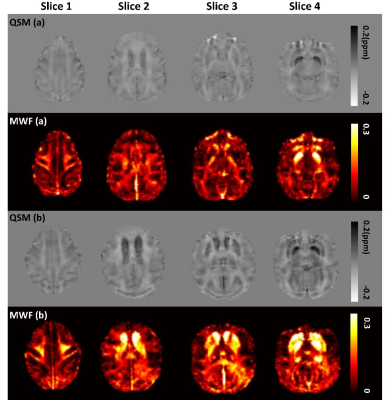
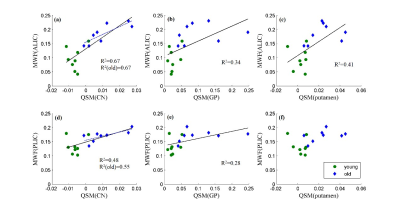
Figure 2 shows the examples of QSM and MWF maps of two representative macaques from young and older groups, respectively. The mean MWF value in the old group was found to be larger than the young group for each WM ROI, but the significant intergroup differences were only found in ALIC (p<0.001) and PLIC (p=0.011). The mean QSM values of the old group were significantly larger than that of the young group in GP (p=0.018), putamen (p=0.002) and CN (p=0.002). Spearman correlation analysis showed that the MWF of ALIC is positively correlated with the QSM of caudate nucleus (r=0.855, p<0.001), globus pallidus (r=0.796, p<0.001) and putamen (r=0.787, p<0.001), and the MWF of PLIC is positively correlated with the QSM of CN (r=0.587, p=0.017) and GP (r=0.620, p=0.01) in the pooled group. In the old group, there are significantly positive correlations between the QSM of CN and the MWF of ALIC (r=0.833, p=0.01) and PLIC (r=0.762, p=0.028). In the young group, no significant correlation between the MWF of WM ROIs and the QSM of BG ROIs was found. Figure 3 shows the scatterplots correlations between the MWF of WM ROIs (ALIC, PLIC) and the QSM of BG ROIs for all macaques.DISCUSSION & CONCLUSION
Our results, from analyses of 16 macaques (8 young + 8 old), show significant and positive associations between the MWF of IC and the QSM of some anatomically connected areas in BG, but no significant correlation was observed between the QSM of BG and the MWF of other WM ROIs anatomically unconnected with BG, except for a negative correlation found between the QSM of GP and the MWF of GCC in the old group. These findings shed lights on the current understanding of the anatomical coupling between iron and myelin. We have demonstrated that moderate to high level of positive correlations exists between the QSM of BG regions and the MWF of anatomically connected IC structures during myelin production and maintenance. These results suggest that future studies should take into account the impact of iron in BG on the myelin of anatomically distinguished yet connected WM structures.Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2017YFC1310400), the Strategic Priority Research Program of Chinese Academy of Science (No. XDB32030000), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX05), grants from the National Natural Science Foundation of China (81871428, 91632109 to JHZ; 81571300, 81527901, 31771174 to ZW) , Natural Science Foundation and Major Basic Research Program of Shanghai (No. 16JC1420100), Shanghai Key Laboratory of Psychotic Disorders(13dz2260500), and Major Scientific Project of Zhejiang Lab (No. 2018DG0ZX01), the Fundamental Research Funds for the Central Universities(2019QNA5026).References
1. Arshad M., Stanley J.A., and Raz N., Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. Neuroimage, 2016. 143: p. 26-39.
2. Wang S. and Young K.M., White matter plasticity in adulthood. Neuroscience, 2014. 276: p. 148-60.
3. Westlye L.T., Walhovd K.B., Dale A.M., et al., Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex, 2010. 20(9): p. 2055-68.
4.Ward R.J., Zucca F.A., Duyn J.H., et al., The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol, 2014. 13(10): p. 1045-60.
5. Hametner S., Endmayr V., Deistung A., et al., The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation - A biochemical and histological validation study. Neuroimage, 2018. 179: p. 117-133.
6. Lv Q., Yang L., Li G., et al., Large-Scale Persistent Network Reconfiguration Induced by Ketamine in Anesthetized Monkeys: Relevance to Mood Disorders. Biol Psychiatry, 2016. 79(9): p. 765-775.
7. Wang Z., Chen L.M., Negyessy L., et al., The relationship of anatomical and functional connectivity to resting-state connectivity in primate somatosensory cortex. Neuron, 2013. 78(6): p. 1116-26.
8. Zhang Z., Cai D.C., Wang Z., et al., Isoflurane-Induced Burst Suppression Increases Intrinsic Functional Connectivity of the Monkey Brain. Front Neurosci, 2019. 13: p. 296.
9. Wu Z., He H., Sun Y., et al., High resolution myelin water imaging incorporating local tissue susceptibility analysis. Magn Reson Imaging, 2017. 42: p. 107-113.
10. Rohlfing T., Kroenke C.D., Sullivan E.V., et al., The INIA19 Template and NeuroMaps Atlas for Primate Brain Image Parcellation and Spatial Normalization. Front Neuroinform, 2012. 6: p. 27.
11. Taljan K.A.I., Investigations of Anatomical Connectivity in the Internal Capsule of Macaques with Diffusion Magnetic Resonance Imaging. ETD Archive. 2011: Cleveland State University. 659.
12. Zarei M., Johansen-Berg H., Jenkinson M., et al., Two-dimensional population map of cortical connections in the human internal capsule. J Magn Reson Imaging, 2007. 25(1): p. 48-54.
Figures