1427
Steady state effects on the myelin water pool: evidence for a distinct T1 compartment?1Research Centre Juelich, Juelich, Germany, 2University of British Columbia, Vancouver, BC, Canada
Synopsis
The influence of steady state on myelin water fraction derived from mGRE data was investigated, with the aim of estimating myelin water T1. Myelin and tissue water were identified by their T2* properties following an NNLS analysis. PCA denoising reduced the variability (SD) of MWF by nearly a factor of 2. The residuals of the NNLS fit were found to be small (below 0.5%) but highly significant, and showed no dependence on TR. The derived T1 of myelin water was highly variable and shorter than that of tissue water. These findings are consistent with a reduced exchange between pools.
Introduction
Myelin is ubiquitous in determining MR contrast in the living brain, especially at high fields, but its quantification by MRI is still under development. In particular, the properties of myelin water (water trapped between myelin bilayers) – e.g. longitudinal relaxation, exchange with other water pools – are insufficiently studied. Multiple-echo gradient echo-based myelin water fraction (mGRE-MWF) determination is a potential alternative to spin-echo methods [1], especially at 3T and higher fields [2,3]. Using gradient echo methods, MWF can be investigated within a large parameter space, allowing the study of more than one property of myelin water within a short measurement time. In the following, we improve the quality of MWF maps by noise reduction using Principal Component Analysis (PCA) and investigate the interesting question of whether T1 saturation effects in the steady state are different in myelin and tissue water.Materials and Methods
Results using a 2D mGRE acquisition were obtained from twelve healthy volunteers (6 females and 6 males, 30±7yo) scanned on a 3T TIM-Trio Siemens scanner. The parameters of the experimental 2D mGRE protocol included: resolution 1x1x2.5mm3, ‘fast pulse’ a= 90o, 32 echoes (TE1= 3.24ms, DTE=1.54ms), TR=2200ms (TA=11:53, 24 slices, 2avgs), 800ms (TA=8:39, 14 slices, 4avgs) or 216 ms (TA=11:42min, 4 slices, 20avgs), 2 repetitions averaged off-line. For a number of 4 volunteers (male, 29±3yo), a single slice acquisition was performed, with all other parameters being kept as above. Data were denoised using Principal Component Analysis [4] and analysed using NNLS with Tikhonov regularisation. T2* intervals were defined based on the relaxometric spectra and were assigned to myelin water, tissue water and CSF-like components. We define robust features in the behavior of the myelin water pool by analyzing the integrated signal from specific ROIs: corpus callosum (CC) and left and right forceps major (LFM, RFM), where the fibres are perpendicular to the B0 field.Results
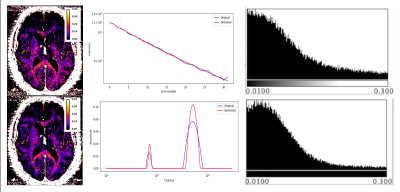
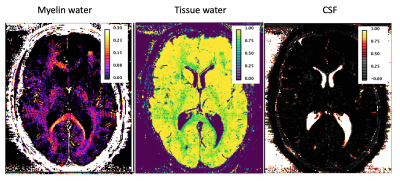
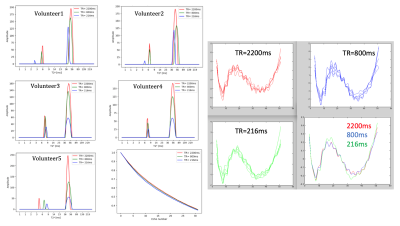
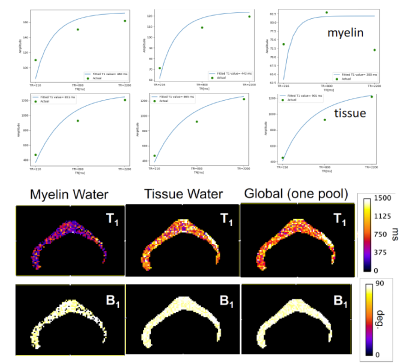
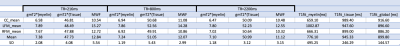
We have selected for the present analysis five out of twelve volunteers, which showed the highest data quality. Fig. 1 shows the effect of noise reduction using PCA. Maps of the different water fractions are displayed in Fig 2 based on the denoised data. The changes in the integrated signal from CC with TR and the changes in its NNLS analysis are shown in Fig. 3a. The residuals of the NNLS fit are shown in Fig. 3b, for all volunteers at a given TR (same colours) as well as TR-specific averaged over all volunteers (red, green, blue). Fig. 4 depicts the results of T1 analysis for the myelin water and tissue water pools separately, as well as for the total signal (‘single pool’). The pertinent changes in the water pools with TR are summarized in Table 1.Discussion and conclusions
PCA-based denoising increases the visual quality of the MWF maps and reduces the SD of the MWF by a factor of 1.7 (Fig.1), similar to [5] for SE data. The results obtained from single and multi-slice measurements at TR=2200ms were not significantly different, indicating negligible effects of incidental magnetization transfer effects, which can be found in multi-slice acquisitions. Assuming that the intensity changes for each water pool can be described by the Ernst equation, the fit to the TR-dependent signals delivers T1 and B1 values (Fig. 4 and Table 1), which are different for myelin, compared to tissue water. The TR-dependent characteristics of the myelin water pool (Table 1) and fit residuals (Fig. 3b) are obtained by averaging over all 5 volunteers and ROIs. The position of the myelin water peak changes with TR for most volunteers and ROIs (Fig. 3a). However, the changes in myelin T2* values with TR (Table 1) are not significant at the group level, since a pronounced variability is observed between volunteers. In contrast, the residuals are highly reproducible over all ROIs and volunteers and describe a very small (below 0.5%) but highly significant effect, as observed before [6]. The behavior of the residuals with TE can be described with a three-compartment complex model [6], and is very sensitive to the off-resonance frequencies of both myelin and axonal water pools. The fit residuals remain practically unchanged with TR (Fig. 3b), which is puzzling if the exchange between myelin and tissue water was large. Indeed, in the presence of exchange, TR-dependent properties of the myelin and tissue pools can be expected due to perturbed equilibrium by different steady states. Chemical exchange between myelin and tissue water, if present, would influence the observed frequency of the effective pools and thus the modulation of the residuals, which is not observed. However, a more complicated exchange picture [7] might change this interpretation. In conclusion, T1 effects in myelin water fraction are observed and described in vivo. The shorter T1 of the myelin water pool is consistent with results obtained very recently using a highly sampled Look-Locker inversion-recovery curve (T1~400ms) [8]. However, the observed short T1 values might be the result of multi-pool exchange rather than corresponding to an individual pool [7]. Finally, whereas mGRE does not yet seem able to compete with mSE for NNLS-based whole-brain, robust MWF analysis, it does offer unique means of studying the properties of water pools in tissue.Acknowledgements
The contribution made by Dr. Sandra Myers in the early stages of this work is very gratefully acknowledged.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 764513.
References
[1] A. MacKay et al., Magn. Reson. Med. 31 (1994); [2] A.M. Oros-Peusquens et al Procs ISMRM 2013; [3] E. Alonso-Ortiz et al. Magn Reson Med. 79 (2018); [4] Veraart et al, Neuroimage 142 (2016); [5] MD Does et al., Magn Reson Med. 81 (2019); [6] P. van Gelderen et al., Magn Reson Med. 67 (2012); [7] Barta et al. J Magn Reson. 259 (2015); [8] A.M. Oros-Peusquens et al Procs ISMRM 2019.Figures