1423
Delayed conscious access assessed by structural connectivity and task-based fMRI using visual backward masking paradigm in Multiple Sclerosis.1Institut für Neuroimmunologie und Multiple Sklerose (INIMS), Universitätsklinikum Hamburg-Eppendorf (UKE), Hamburg, Germany, 2CRMBM AMU-CNRS , Marseille, France, Marseille, France, 3CEMEREM, APHM, CHU Timone, Marseille France, Marseille, France, 4Klinik und Poliklinik für Neurologie, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany, 5Charité - Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt Universität zu Berlin, and Berlin Institute of Health (BIH), Klinik für Psychiatrie & Psychotherapie und Medizinische Klinik m.S. Psychosomatik, Campus Benjamin Franklin (CBF), Berlin, Germany
Synopsis
Visual backward masking paradigm (VB) distinguishes between conscious and unconscious processes and evaluates fundamental brain functions and cognition. Delayed consciousness was shown as related with white matter damage in early Multiple Sclerosis (MS). However, it is unknown, if regional brain activity and structural connectivity are related to delayed consciousness in MS. We investigated the impaired access to consciousness in MS[25(MS)/37(controls)] using task-based-fMRI based on the VB and its relation with structural connectivity. Lower activation was detected in unconscious-processing in MS vs controls. Strong relationship between VB-performance and lower structural connectivity in ventral-attention/visual/default-mode networks supports the sensitivity of VB in MS.
Purpose
Cognitive impairment is common in Multiple Sclerosis (MS)1. Visual backward masking that distinguishes between conscious and unconscious processing is an established paradigm in cognitive neuroscience2. In the paradigm, when there is a delay between a fast stimuli followed by a backward mask, subjects could not perceive the stimuli until the delay exceeds 50 msec3. Previously, the regions in the human cortex that are related to unconscious and conscious perception have been demonstrated in healthy controls (HC)4 and schizophrenia patients5 using task-based functional magnetic resonance imaging (fMRI). In addition, the impaired access to consciousness has been shown as related to white matter damage in early MS6. However, it is unknown if the regional activity is altered based on the paradigm in MS. Therefore, we aimed to investigate the impaired access to consciousness in MS using task-based fMRI based on the visual backward masking paradigm. Furthermore, we hypothesized that altered structural connectivity in MS would be related to the objective performance of the visual backward masking paradigm.Methods
IRB was taken and all participants gave written informed consent.Subjects: The study included 25 MS patients [(15 Relapsing-Remitting/7 Primary-Progressive/3 Secondary-Progressive MS) median disease duration=7 (range=1-20) year, median Expanded Disability Status Scale=2.5 (range=1.5-6)] and 37 age-sex-education matched HC (p>0.05).
MRI protocol: All participants underwent 3T (Siemens/Prisma) and the protocol included diffusion tensor imaging (single shell; 32 direction; b=1000s/mm2; TR/TE=7200/90msec; FOV=240mm; matrix=128x128; voxel size=1.9×1.9×2.0mm; 54 axial-sections; no gap), and 3D T1-weighted (TR/TE=1800/2.3msec; FA=8; FOV=250mm; matrix=288x288) sequences. Functional multiband (MB) echo planar imaging (MB=2; TR/TE=2000/30msec; 60 slices; voxel size=2x2x2mm; FOV=224mm×224mm; matrix=112x112) was used for task-based fMRI.
Task-based fMRI design: The target was a number (1, 4, 6 or 9) and was presented for 16 msec in one of four locations relative to the central fixation point. The mask that included another number (1, 4, 6 or 9) and 3 letters (E, M, E) surrounded the area of the target and was presented for 150 msec at one of eight possible stimulus onset asynchrony (SOA)s: 8, 16, 33, 50, 66, 83, 100, 150 msec. Subjects were asked to compare the target with 5. Each trial took 2 sec, had a fixed answering time and each block for each SOA involved 40 trials.
Behavioral data: The inflection point, that is the SOA where the accuracy suddenly increases and participants switch from unconscious to conscious processing, was calculated by Taylor regression7.
Task-based fMRI analysis: Slice timing, realignment, co-registration and spatially normalization to Montreal Neurological Institute anatomical template were performed using SPM128. First level analysis of each subject included 3 experimental conditions as consciousness (SOAs>50msec), unconsciousness (SOAs<50msec) and resting. Then, the group differences of conditions were extracted by two-sampled t-test.
Structural connectivity: Structural connectivity based on probabilistic tractography was extracted using MRITRIX39 as described in Beeson et al10. After lesion filling, automated procedure for the volume measurement was performed using Freesurfer11. Finally, gray-matter parcellation of 80 regions (total=160) for each hemisphere was specified based on the Destrieux atlas12 to perform the structural connectivity analysis. The location of each node in one of seven main functional sub-networks (Yeo atlas) was determined on the FreeSurfer fsaverage13.
Results
Objective performance obtained during FMRI revealed that MS needed longer SOA to access consciousness and reached a lower accuracy rate than HC (Fig.1). When we compared the unconscious processes (SOA<50msec), there were widespread decreased activations in the bilateral precuneus and middle temporal, right angular, posterior cingulate gyrus and hippocampus, left lingual, middle cingulate and superior temporal gyrus in patients compared HC (p<0.05,FWE) (Fig.2). In contrast, in conscious processes (SOA>50msec), there was only a decrement in the left angular gyrus in patients compared to HC (Fig.3). When we compared the difference in brain activation between conscious and unconscious processing, we observed a decreased activation in the left superior parietal and right inferior temporal gyrus in MS (Fig.4). The association between global structural connectivity and the infection point differed significantly between MS and HC (p=0.00893) such as we observed a tendency for an inverse correlation in patients while it was positively correlated in HC (r=0.35, p=0.033) (Fig.5a). Analyzing the same metric for seven Yeo networks, higher nodal structural connectivity was correlated with lower behavioral inflection point mainly in ventral attention, default-mode and visual networks in MS (r>-0.4, p<0.001) while HC showed consistently an inverse correlation (Fig.5b).Discussion and Conclusion
We detected lower activation in brain regions that are sensitive to masking effect in MS patients compared to HC and it was pronounced in unconscious processing. These regions were mentioned as major for masking effect in HC4. Moreover, this impaired performance in is related to a loss of structural integrity of certain functional networks known to be important for cognitive functioning. Thus, the visual backward masking paradigm assessed by task-based fMRI seems to be sensitive to the detection of impaired unconscious processing in MS. This is highly relevant in MS as conceptually unconscious processing is a fundamental feature which might be related to several MS impairments such as attention, social cognition or fatigue. This paradigm may provide a powerful tool to further explore the development and impact of delayed access to conscious processing on other bodily functions during the course of MS.Acknowledgements
No acknowledgement found.References
1. Hansen S, Muenssinger J, Kronhofmann S, Lautenbacher S, Oschmann P, Keune PM. Cognitive screening tools in multiple sclerosis revisited: Sensitivity and specificity of a short version of Rao’s Brief Repeatable Battery. BMC Neurol. 2015;15(1):9-11. doi:10.1186/s12883-015-0497-8
2. Del Cul A, Baillet S, Dehaene S. Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol. 2007;5(10):e260. doi:10.1371/journal.pbio.0050260
3. Del Cul A, Baillet S, Dehaene S. Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol. 2007;5(10):2408-2423. doi:10.1371/journal.pbio.0050260
4. Green MF, Glahn D, Engel SA, et al. Regional brain activity associated with visual backward masking. J Cogn Neurosci. 2005;17(1):13-23. doi:10.1162/0898929052880011
5. Green MF, Lee J, Cohen MS, et al. Functional neuroanatomy of visual masking deficits in schizophrenia. Arch Gen Psychiatry. 2009;66(12):1295-1303. doi:10.1001/archgenpsychiatry.2009.132
6. Reuter F, Del Cul A, Malikova I, et al. White matter damage impairs access to consciousness in multiple sclerosis. Neuroimage. 2009;44(2):590-599. doi:10.1016/j.neuroimage.2008.08.024
7. Christopoulos D. Roots, extrema and inflection points by using a proper Taylor regression procedure. SSRN Electron J. 2014. doi:10.2139/ssrn.2521403
8. Ashburner J, Barnes G, Chen C, et al. SPM12 Manual The FIL Methods Group ( and honorary members ). Funct Imaging Lab. 2013:475-1. doi:10.1111/j.1365-294X.2006.02813.x
9. Tournier J-D, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. doi:10.1016/J.NEUROIMAGE.2019.116137
10. Besson P, Dinkelacker V, Valabregue R, et al. Structural connectivity differences in left and right temporal lobe epilepsy. Neuroimage. 2014;100:135-144. doi:10.1016/j.neuroimage.2014.04.071
11. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341-355. http://www.ncbi.nlm.nih.gov/pubmed/11832223. Accessed November 13, 2018.
12. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1-15. doi:10.1016/j.neuroimage.2010.06.010
13. Thomas Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125-1165. doi:10.1152/jn.00338.2011
Figures
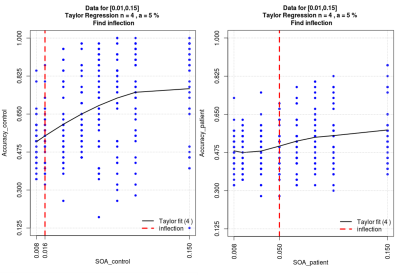
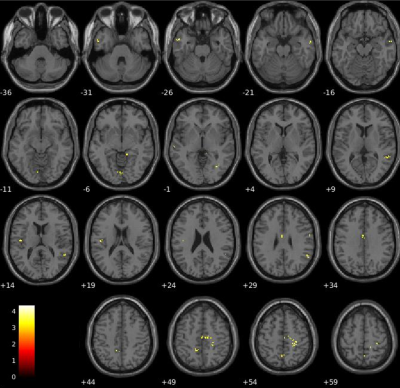
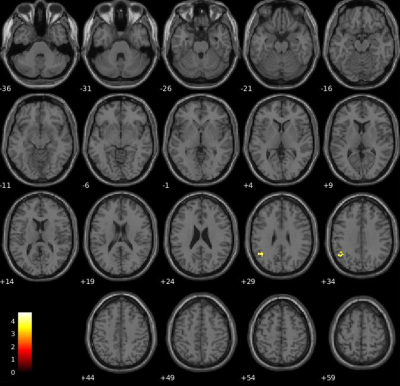
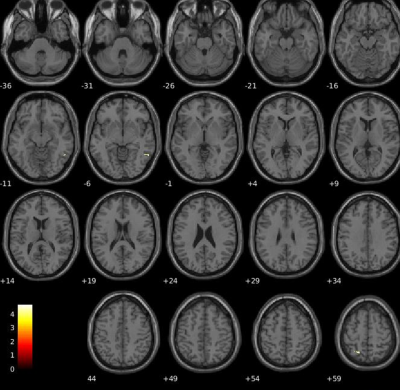
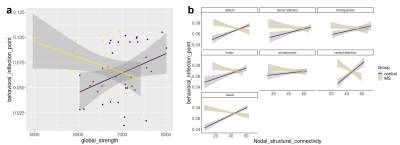
Figure 5. Figure shows the relationship between the behavioral inflection point (the time needed to access consciousness calculated by Taylor regression of objective performance in comparison of target number with 5 as a function of SOA) of each subject and global structural connectivity (a) / nodal structural connectivity in each subnetwork based on the Yeo atlas (b) in patients (yellow) and controls (purple).
(Structural connectivity calculated based on graph strength).