1410
Optimized patient-specific refocusing control in turbo spin echo FLAIR for improved detection of infratentorial lesions in multiple sclerosis1Diagnostic and Interventional Imaging, University of Texas Health Science Center at Houston, Houston, TX, United States, 2Philips healthcare, Gainesville, FL, United States, 3Neurology, University of Texas Health Science Center at Houston, Houston, TX, United States
Synopsis
Detection of infratentorial lesions is important for diagnosis of multiple sclerosis. However, infratentorial lesions are hard to detect on conventional FLAIR protocols. In this work, we optimized the refocusing flip angle in the turbo spin echo readout of 3D FLAIR to enhance infratentorial lesion contrast in individual patients. T1, T2, and proton density measured in the brain stem and cortical gray matter were used to calculate the refocusing angle that maximizes the contrast-to-noise ratio. The patient-specific approach was assessed in 8 MS patients. Improved infratentorial lesion contrast was achieved in most of the cases.
Introduction
MRI plays a key role as the primary noninvasive imaging modality in multiple sclerosis (MS). Infratentorial lesions provide MRI evidence for the ‘dissemination in space’ criterion for diagnosing MS.1 Infratentorial lesion volume correlated with the sensory functional system score2, and are thought to be predictive of long-term prognosis for patients with initial findings suggestive of MS.3 The number and volume of infratentorial T1 hypointense lesions correlated with EDSS score in MS patients with chronic cerebellar ataxia.4Fluid-attenuated inversion recovery (FLAIR) images are sensitive to MS hyper-intense lesions. However, infratentorial lesions are more difficult to detect on FLAIR. We hypothesized that the standard scan parameters, optimized for general brain abnormalities, are less sensitive for the more subtle contrast of infratentorial lesions. Recent studies have shown better sensitivity to infratentorial lesion using optimized 3D FLAIR protocol.5 Other work suggested the potential advantage of optimizing scan parameters on the patient level.6 In this study, we investigated use of patient-specific optimization of FLAIR for enhancing the lesion-white matter contrast of infratentorial lesions.
Methods
Eight MS patients were recruited for this study. In each patient, T1 mapping was performed using the Look-Locker sequence through a single coronal slice (voxel dimension = 1.2×1.2×3 mm3) passing through the brain stem (TR/TE = 6.7/2.8 ms. Flip angle = 7°, number of phases = 65, cycle interval = 6000 ms). T2 and proton density (PD) mapping was performed in the same slice using multi-echo (n=16) spin echo protocol (TR/TE1/ΔTE = 3000/16/8 ms). Immediately after acquisition, the data were exported to the analysis workstation, where T1, T2, and PD maps were rapidly computed using the graphical pipeline environment (GRAPE) software.7 Two regions of interest were manually drawn in the pons and cortical gray matter. The mean tissue parameters were computed from the ROIs and used to optimize FLAIR. The flip angle of the refocusing pulses in the long turbo spin echo train was used as the control parameter to optimize image contrast. 3D FLAIR was acquired with the following scan parameters: scan plane, sagittal; FOV = 256×256×180 mm3; voxel size = 1×1×1 mm3; TR/TI/TE=4800/1650/300 ms; turbo spin echo factor, 167 (6 startup pulses); scan time 5:31 min. The contrast-to-noise ratio (CNR) between cortical gray matter and the pons was used as the objective function for optimization. For comparison, the FLAIR acquisition was repeated with identical scan parameters but with the default refocusing angle of 40°. Other than manually drawing the ROIs, the processing framework was fully automated, including the export of the images from the scanner database, data transfer to the analysis workstation, relaxation time calculation, scan optimization, importing the optimized scan parameter, and applying the optimized parameter in the scan protocol using a custom programmed environment into the scanner.Results
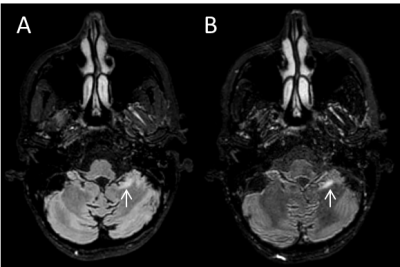
As an example, Figure 1 shows FLAIR images acquired with the default and optimized refocusing flip angles. The improved lesion contrast in the infratentorial region can be readily appreciated from this figure. Overall, 10 infratentorial lesions were seen in seven out of the eight patients on the FLAIR images. A trend of higher CNR for the infratentorial lesions was observed on the optimized FLAIR acquisition compared to the default FLAIR (10.3±6.1 vs. 8.4±3.1, P=0.24). Scan optimization was successfully achieved during the same scan session in all subjects. Turn-around time was 2-3 minutes, with most of that time spent in generating the tissue parameter maps.Discussion
The results suggest that better infratentorial lesion contrast may be obtained with patient-specific optimization of FLAIR. Adjusting the refocusing pulses allowed fine control of contrast without changing the protocol timing, and with consistent fluid suppression. Radiofrequency field (B1) inhomogeneity can be problematic for this approach if it causes large deviations from the nominal flip angles. Those variations can also cause inaccuracies in the estimated relaxation times and proton density. Future work will include additional B1 field map for correction of these effects. Automated ROI selection can also be implemented for faster execution and to avoid possible operator bias in ROI placement. The study shows promising results, and further qualitative and quantitative evaluation will be conducted in a future study on a larger cohort.Conclusion
Adapting the scan parameter to each individual patient provides improved lesion contrast, which could enhance the detection of infratentorial lesions in MS patients.Acknowledgements
We thank Vipulkumar Patel and Corina Donohue for help with MRI data acquisition.References
1. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, others. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173.
2. Quattrocchi CC, Cherubini A, Luccichenti G, Sabatini U, Grasso MG, Nocentini U, Beomonte Zobel B. Infratentorial lesion volume correlates with sensory functional system in multiple sclerosis patients: A 3.0-Tesla MRI study. Radiol Medica. 2010;
3. Minneboo A, Barkhof F, Polman CH, Uitdehaag BMJ, Knol DL, Castelijns JA. Infratentorial Lesions Predict Long-term Disability in Patients with Initial Findings Suggestive of Multiple Sclerosis. Arch Neurol. 2004;
4. Hickman SJ, Brierley CMH, Silver NC, Moseley IF, Scolding NJ, Compston DAS, Miller DH. Infratentorial hypointense lesion volume on T1-weighted magnetic resonance imaging correlates with disability in patients with chronic cerebellar ataxia due to multiple sclerosis. J Neurol Sci. 2001;
5. Lecler A, El Sanharawi I, El Methni J, Gout O, Koskas P, Savatovsky J. Improving Detection of Multiple Sclerosis Lesions in the Posterior Fossa Using an Optimized 3D-FLAIR Sequence at 3T. Am J Neuroradiol. 2019;
6. Gabr RE, Pednekar AS, Govindarajan KA, Sun X, Riascos RF, Ramirez MG, Hasan KM, Lincoln JA, Nelson FM, Wolinsky JS, Narayana PA. Patient-specific 3D FLAIR for enhanced visualization of brain white matter lesions in multiple sclerosis. J Magn Reson Imaging. 2017;46:557–564.
7. Gabr RE, Tefera GB, Allen WJ, Pednekar AS, Narayana PA. GRAPE: a graphical pipeline environment for image analysis in adaptive magnetic resonance imaging. Int J Comput Assist Radiol Surg. 2017;12:449–457.