1402
Reduced field-of-view multi-shell diffusion-weighted imaging of the sciatic nerve: Application to multiple sclerosis1Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, United Kingdom, 2Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom, 3Philips Healthcare, Guildford, Surrey, United Kingdom, 4Philips Japan, Minatoku, Tokyo, Japan, 5Centre for Medical Image Computing, Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 6Universitat Oberta de Catalunya, Barcelona, Spain, 7Brain MRI 3T Research Centre, IRCCS Mondino Foundation, Pavia, Italy, 8Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy
Synopsis
Ex vivo investigations have demonstrated the involvement of the peripheral nervous system in multiple sclerosis (MS). However, the use of advanced imaging methods to study individual peripheral nerves in vivo, such as the sciatic nerve, has been hindered by a number of technical challenges. In this pilot in vivo study, we explore the feasibility of acquiring multi-shell diffusion-weighted imaging (DWI) metrics in the sciatic nerve of people with relapsing-remitting MS (RRMS) using reduced field-of-view echo planar imaging. DWI metrics in people with RRMS display changes in comparison to healthy controls, suggesting that the structural integrity of the nerve is compromised.
INTRODUCTION
Neuropathological and biopsy reports have demonstrated the involvement of the peripheral nervous system (PNS) in multiple sclerosis (MS), with demyelination and axonal degeneration being the dominant underlying pathophysiological processes involved1-3. As such, it is conceivable that the use of advanced microstructural diffusion-weighted imaging (DWI) methods, such as diffusion kurtosis imaging (DKI) or neurite orientation dispersion and density imaging (NODDI), which probe tissue microstructure non-invasively, would be relevant in the context of PNS involvement in MS. In this pilot in vivo study, we explore the feasibility of acquiring multi-shell DWI metrics in the sciatic nerve using reduced field-of-view echo planar imaging (ZOOM-EPI) as new approach to alleviate some of the common technical problems associated with conventional single-shot EPI, such as image distortions in the presence of magnetic susceptibility due to the long readout duration, and we report preliminary findings in a small number of people with MS.METHOD
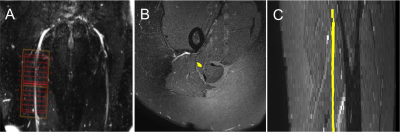
1) Participants: Five healthy controls (mean age 33.6 years, 3 female, range 30-38) and three people with relapsing remitting MS (RRMS) (mean age 42.3 years, 2 female, range 36-53) were recruited. Informed consent was obtained from all study participants and the study was approved by local ethics; 2) MR imaging: Using a Philips Ingenia CX 3T with 28-channel anterior and posterior coils, the sciatic nerves were first located using a 3D SHINKEI sequence with a large FOV4,5; these images were subsequently used to facilitate planning of high-resolution fat-suppressed T2-weighted and ZOOM-EPI acquisitions in the axial plane (i.e. perpendicular to the longitudinal axis of the nerve) for segmentation of the sciatic nerve and calculation of the DWI metrics, respectively (Figure 1A). The acquisition parameters for the 3D SHINKEI sequence were as follows: TR = 2200 ms; TE = 180 ms, FOV = 300 × 420 mm2, voxel size = 1.2 x 1.2 x 2 mm3, number of averages = 1, TSE factor = 56, improved motion-sensitized driven-equilibrium (iMSDE) duration = 50 ms, 170 slices, scanning time of 05:43 min. The acquisition parameters for the fat-supressed T2-weighted acquisition were as follows: TR = 5000 ms; TE = 60 ms, FOV = 180 × 180 mm2, voxel size = 0.5 x 0.5 x 4 mm3, number of averages = 1, TSE factor = 11, 30 slices, scanning time of 08:08 min. The multi-shell DWI acquisition was planned with identical scan geometry to the fat-supressed T2-weighted acquisition as follows: TR = 6300 ms; TE = 66 ms; FOV = 64 × 48 mm2; voxel size = 1 x 1 x 10 mm3; number of averages = 2; half-scan= 0.6; 12 slices; b=1000 s/mm2, 22 directions; b=2000 s/mm2, 21 directions; b=3000 s/mm2; 4 interleaved non-diffusion-weighted (b=0) images were also acquired; scanning time was 17:31 min; 3) Image analysis: The sciatic nerve was manually segmented by one experienced rater in FSLview (http://www.fmrib.ox.ac.uk/fsl/) using the fat-suppressed T2-weighted scan (Figures 1B and 1C). All DWI images were registered to the fat-suppressed T2-weighted image using affine registration with NiftyReg7. We fitted the DKI signal representation to the acquired multi-shell DW data using the DiPy dkifit (https://dipy.org/documentation/1.0.0./examples_built/reconst_dki/)6,7;the fitting provided standard diffusion tensor metrics fractional anisotropy (FA), axial/radial/mean diffusivity (AD/RD/MD) as well as DKI metrics axial/radial/mean kurtosis (AK/RK/MK). For the NODDI fitting, the Matlab (The MathWorks, Inc., Natick, Massachusetts, USA) NODDI toolbox (http://nitrc.org/projects/noddi_toolbox) was used to generate orientation dispersion index (ODI) and neurite density index (NDI) maps8. ODI measures the variability in axonal orientations within a voxel, higher as ODI approaches 1, while NDI provides a surrogate measure of axonal density.RESULTS
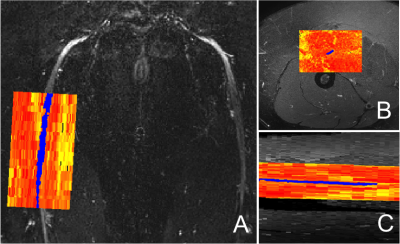
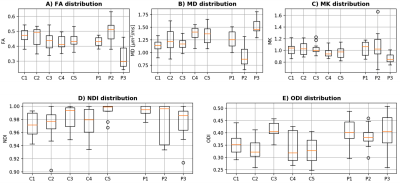
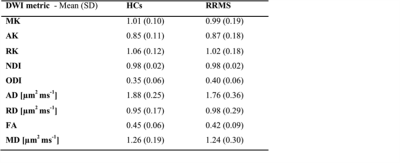
In a 12 cm section of the sciatic nerve (mid thigh; right leg) of people with RRMS, mean (SD) MK, ODI, NDI, FA and MD were 0.99 (0.19), 0.40 (0.06), 0.98 (0.02), 0.42 (0.09) and 1.24 (0.30) µm2 ms-1, respectively, and in HCs were 1.01 (0.10), 0.35 (0.06), 0.98 (0.02), 0.45 (0.06) and 1.26 (0.19) µm2 ms-1, respectively. Table 1 shows the results of all DWI metrics calculated in all study participants. Figure 2 shows an example of the FA map calculated in the sciatic nerve of a healthy control and Figure 3 shows the distribution of DWI metrics FA, MD, MK, NDI, ODI in all study participants.DISCUSSION AND CONCLUSION
In this pilot study, we have demonstrated the feasibility of obtaining multi-shell DWI metrics in the sciatic nerve of healthy controls and people with RRMS using ZOOM-EPI. Additionally, we have demonstrated changes in the mean DWI metrics of people with RRMS as compared to HCs, displaying trends that are consistent with data from histopathological studies in the literature. Specifically, a reduction in MK and FA of people with RRMS with a concomitant increase in ODI, are consistent with reports of demyelination and axonal degeneration in the peripheral nerves of people with MS. Future investigations using a larger sample population will be required to confirm the results presented in this pilot study.Acknowledgements
The UK MS Society and the UCL-UCLH Biomedical Research Centre for ongoing support. CGWK receives funding from ISRT, Wings for Life and the Craig H. Neilsen Foundation (the INSPIRED study), from the MS Society (892/08 and 77/2017), Wings for Life (#169111), Horizon2020 (CDS-QUAMRI, #634541). FP is a non-clinical Postdoctoral Guarantors of Brain fellow. This project has received funding under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 634541 and and from the Engineering and Physical Sciences Research Council (EPSRC EP/R006032/1), funding FG.References
1) Hasson J, Terry R.D Zimmerman H.M. Peripheral neuropathy in multiple sclerosis. Neurology. 1958; 8(7):503-10.
2) Miglietta O, Lowenthal M. A study of peripheral nerve involvement in fifty-four patients with multiple sclerosis. Arch Phys Med Rehabil. 1961;42:573-8.
3) Pollock M, Calder C, Allpress S. Peripheral nerve abnormality in multiple sclerosis. Ann Neurol. 1977;2(1):41-8.
4) Yoneyama M, Takahara T, Kwee T.C, et al. Rapid high resolution MR neurography with a diffusion-weighted pre-pulse, Magn. Reson. Med. Sci. 2013;12(2):111–119.
5) Kasper J.M, Wadhwa V, Scott K.M, et al. SHINKEI-a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging, Eur. Radiol. 2015;25(6):1672–1677.
6) Hansen, B, Jespersen, S.N. Data for evaluation of fast kurtosis strategies, b-value optimization and exploration of diffusion MRI contrast. Sci Data. 2016;3:160072. doi:10.1038/sdata.2016.72
7) Garyfallidis E, Brett M, Amirbekian B, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform. 2014;8:8.doi: 10.3389/fninf.2014.00008. eCollection 2014.
8) Zhang H, Schneider T, Wheeler-Kingshott CA, et al. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–1016.
Figures