1396
Diffusion tractography evaluation of specificity of PASAT and SDMT to cognitive pathway in multiple sclerosis1Imaging Institute, Cleveland Clinic, Cleveland, OH, United States, 2Radiology, Cleveland Clinic Lerner College of Medicine, CLEVELAND, OH, United States, 3Neurological Institute, Cleveland Clinic, Cleveland, OH, United States, 4Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Paced auditory serial addition test (PASAT) and symbol digit modalities test (SDMT) are two most commonly used tests to evaluate cognitive performance in MS. The specificity of the two tests to cognitive network in brain was evaluated with diffusion tensor imaging and correlating transverse diffusivity (TD) along different functionally relevant pathways with PASAT/SDMT scores. While SDMT showed association with TD along all pathways (and whole brain white matter), PASAT was associated with only frontoparietal pathway, establishing its specificity to cognitive network.
INTRODUCTION
Tractography along functionally relevant pathways using diffusion tensor imaging (DTI) is a powerful tool to associate different functionalities with neural networks. Clinical evaluation of patients for functional impairment requires task specific tests to adequately address disability. Cognitive impairment is a common symptom in multiple sclerosis (MS). 1-3 Paced auditory serial addition test (PASAT)4 and symbol digit modalities test (SDMT)5 are two most commonly used tests to evaluate cognitive performance in MS. In this study we have investigated the specificity of the two tests to cognitive network in brain. For this purpose white matter integrities in MS along frontoparietal pathway (associated with cognition), corticospinal tract (CST, associated with lower limb function), transcallosal motor pathway (associated with upper limb function) and averaged over whole brain white matter, as measured by diffusion tensor imaging (DTI), were correlated with PASAT and SDMT.METHODS
Twenty five patients with MS (age: 42.0±8.6, 10 male) were scanned using a 3T whole body Siemens Tim Trio scanner (Siemens Healthcare, Erlangen, Germany) and standard 12 channel head coil under an IRB-approved protocol. Whole brain DTI, resting state functional connectivity MRI (fcMRI) and anatomical T1-weighted images of each subject were acquired. High angular resolution diffusion imaging (HARDI) protocol with scan parameters 2 mm isotropic, 71 diffusion-weighting gradients with b=1000s/mm2 and 8 b=0 volumes, NEX=4 was used for DTI acquisition. For PASAT, the number of correct responses of addition of two consecutive numbers in 60 seconds was recorded for each subject. For SDMT, following a reference key, subjects were asked to pair specific numbers and digits; the correct number of pairings in 90 seconds was recorded for each subject. Postprocessing of DTI data consisted of the following steps: (i) Motion correction using an iterative approach6 that accounted for eddy current distortions and updated gradient vectors, (ii) the diffusion tensor calculation on a voxel-by-voxel basis accounting for noise floor effects,7 (iii) the fiber orientation distribution8 was calculated in each voxel to inform probabilistic tractography9 to determine white matter pathway-based measures of tissue microstructure. For frontoparietal tracking, right middle frontal gyrus (rMFG) region of interest (ROI) was used as seeds while right inferior parietal lobule (rIPL) ROI of each subject was used as the target. For CST tracking, bilateral primary motor cortex (M1) ROI was used as seed and corresponding ipsilateral cerebral peduncle ROI was used as the target. The left and right M1 ROIs were used as seed and target respectively for transcallosal motor pathway tracking. The (i) rMFG and rIPL frontoparietal and (ii) left and right M1 transcallosal motor pathway ROIs were identified from the processed fcMRI data (not described here) by identifying the areas of maximum correlation using Using AFNI10 tool InstaCorr,11 while the cerebral peduncle was delineated manually on the color fractional anisotropy (FA) map. Transverse, longitudinal and mean diffusivity (TD, LD and MD respectively) as well as fractional anisotropy (FA) along frontoparietal tract, CST and trranscallosal motor pathway were determined. Average DTI metrics over whole brain white matter (WBWM) were also determined. Association between DTI metrics and PASAT/SDMT were determined by Spearman correlation analyses.RESULTS AND DISCUSSION
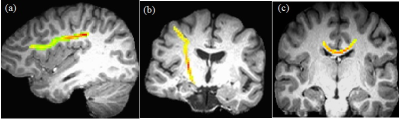
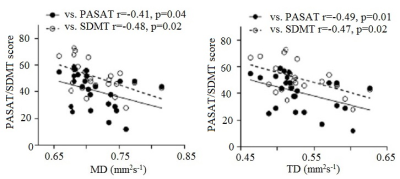
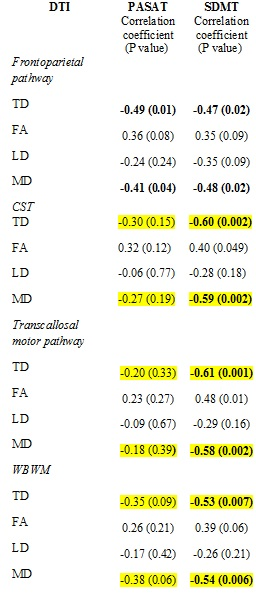
Representative single subject frontoparietal, CST and transcallosal motor pathway tracts are shown in Fig. 1. Correlation of all DTI metrics with PASAT/SDMT are shown in Table 1. While PASAT showed a strong negative correlation with TD/MD along frontoparietal pathway, SDMT was strongly and negatively correlated with TD/MD along frontoparietal, CST, transcallosal motor pathway and WBWM. Plots of SDMT/PASAT scores as functions of frontoparietal TD/MD are shown in Fig. 2. In addition, FA along CST showed moderate association with SDMT. As an increase in TD/MD is indicative of loss in white matter integrity, the data suggest that PASAT is sensitive to white matter injury only along the frontoparietal pathway, whereas SDMT is sensitive to white matter injury along other pathways as well. Previous studies have shown correlation of SDMT with FA in corpus callosum,12, 13 decreased FA / increased TD / LD / MD in the superior longitudinal fasciculus and posterior thalamic radiation13, 14, and in the external capsule, cingulum, sagittal stratum, fornix, uncinate fasciculus, corona radiata, internal capsule, and cerebral peduncle.14 Thus correlation between SDMT and DTI measures in different regions in the brain supports non-specificity of SDMT on any specific pathways. This study establishes better specificity of PASAT to frontoparietal pathway by obtaining PASAT, SDMT and relevant DTI measures from the same group of patients in a single comprehensive study. Also, even though SDMT scores are less affected15 by practice effect than PASAT, 16, 17 and has been suggested to perform better in assessing cognitive impairment than PASAT,18 this study demonstrates better specificity of PASAT to cognitive network in MS.CONCLUSION
Association of cognitive and DTI measures show that PASAT is more specific to cognitive pathway than SDMT. This indicates that PASAT is a more appropriate cognition performance specific task in MS.Acknowledgements
We are grateful to Novartis for funding this project. We thank Thorsten Feiweier of Siemens Healthineers for developing the DTI pulse sequence and the monopolar+ functionality that was used in this study.References
1. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502-1517.
2. Benedict RHB, Holtzer R, Motl RW, Foley FW, Kaur S, Hojnacki D, Weinstock-Guttman B. Upper and Lower Extremity Motor Function and Cognitive Impairment in Multiple Sclerosis. Journal of the International Neuropsychological Society. 2011;17(4):643-653.
3. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurology. 2008;7(12):1139-1151.
4. Gronwall DMA. Paced Auditory Serial-Addition Task - Measure of Recovery from Concussion. Perceptual and Motor Skills. 1977;44(2):367-373.
5. Smith A. Symbol Digits Modalities Test: Manual. West Psychol Sci. 1982:Los Angeles Western Psychological Services.
6. Sakaie KE, Lowe MJ. Quantitative assessment of motion correction for high angular resolution diffusion imaging. Magn Reson Imaging. 2010;28(2):290-296.
7. Sakaie K, Lowe M. Retrospective correction of bias in diffusion tensor imaging arising from coil combination mode. Magn Reson Imaging. 2017;37:203-208.
8. Sakaie KE, Lowe MJ. An objective method for regularization of fiber orientation distributions derived from diffusion-weighted MRI. Neuroimage. 2007;34(1):169-176.
9. Lowe MJ, Beall EB, Sakaie KE, Koenig KA, Stone L, Marrie RA, Phillips MD. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum Brain Mapp. 2008;29(7):818-827.
10. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162-173.
11. Cox R, Saad Z. InstaCorr in AFNI and SUMA. Second Biennial International Conference on Resting-State Functional Brain Connectivity. Milwaukee, WI; 2010.
12. Bethune A, Tipu V, Sled JG, Narayanan S, Arnold DL, Mabbott D, Rockel C, Ghassemi R, Till C, Banwell B. Diffusion tensor imaging and cognitive speed in children with multiple sclerosis. J Neurol Sci. 2011;309(1-2):68-74.
13. Mazerolle EL, Wojtowicz MA, Omisade A, Fisk JD. Intra-individual variability in information processing speed reflects white matter microstructure in multiple sclerosis. Neuroimage Clin. 2013;2:894-902.
14. Yu HJ, Christodoulou C, Bhise V, Greenblatt D, Patel Y, Serafin D, Maletic-Savatic M, Krupp LB, Wagshul ME. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage. 2012;59(4):3713-3722.
15. Benedict RH, Duquin JA, Jurgensen S, Rudick RA, Feitcher J, Munschauer FE, Panzara MA, Weinstock-Guttman B. Repeated assessment of neuropsychological deficits in multiple sclerosis using the Symbol Digit Modalities Test and the MS Neuropsychological Screening Questionnaire. Mult Scler. 2008;14(7):940-946.
16. Barker-Collo SL. Within session practice effects on the PASAT in clients with multiple sclerosis. Arch Clin Neuropsychol. 2005;20(2):145-152.
17. Bever CT, Jr., Grattan L, Panitch HS, Johnson KP. The Brief Repeatable Battery of Neuropsychological Tests for Multiple Sclerosis: a preliminary serial study. Mult Scler. 1995;1(3):165-169. 18. Lopez-Gongora M, Querol L, Escartin A. A one-year follow-up study of the Symbol Digit Modalities Test (SDMT) and the Paced Auditory Serial Addition Test (PASAT) in relapsing-remitting multiple sclerosis: an appraisal of comparative longitudinal sensitivity. BMC Neurol. 2015;15:40.
Figures