1393
Diffusion Tensor Imaging on Three Pathways: Effect of Ibudilast in Progressive Multiple Sclerosis1Imaging Institute, U-15, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Diffusion Tensor Imaging (DTI) analysis was performed in the multi-site
phase II trial of ibudilast in progressive multiple sclerosis. This study
extends that analysis to three pathways to examine DTI measures in 31
progressive MS patients on treatment and 31 patients on placebo. Probabilistic
tracking maps were generated. Mean pathway diffusion measures: longitudinal
diffusivity (LD) and transverse diffusivity (TD) are reported. We report here
that diffusion measures don’t significantly change over time in the treatment
group but we observe a significant change over time in the placebo group.
Introduction
Ibudilast showed slower progression of brain atrophy than placebo in a phase 2 trial for patients with progressive multiple sclerosis (MS).1 In the same trial, Diffusion Tensor Imaging (DTI) was conducted, with the protocol-defined outcome focusing on corticospinal tracts (CST). In this study, we examine DTI measures in the two additional pathways: the optic radiations (OR) and the Papez circuit (PC). These pathways are of interest because they are involved with visual and memory function, which are commonly affected in MS.Method
Data CollectionUnder an IRB-approved protocol, 255 subjects were enrolled in multi-site phase II trial of ibudilast in progressive multiple sclerosis.1 This analysis analyzed images from a subset of 62 subjects who completed all timepoints on the same scanner (Siemens Trio (Siemens Healthineers Erlangen, Germany)). 31 subjects were assigned to Ibudilast and 31 to placebo. Patients were scanned at five time points: baseline, 24, 48, 72 and 96 weeks. Anatomical T1-weighted images were collected with voxel size of 1mm isotropic. DTI scans were conducted with scan parameters: TR/TE = 7400ms/80ms, 60 slices, voxel size = 2.5mm isotropic, matrix = 102x102, 64 b = 700 sec/mm2 and 8 b=0.
Data Analysis
TORTOISE was used for motion and eddy current distortion correction.2 Images from each time point were co-registered with AFNI.3 At the right side of brain, regions of interest were manually drawn on precentral gyrus and peduncle to define the CST, lateral geniculate nucleus and primary visual cortex for the OR, and posterior cingulate and entorhinal cortex for the PC. Probabilistic tracking maps were generated along three pathways with probablistic tractograph.4 Weighted means of DTI measures were calculated along each pathway.4 The mean, across subjects, of TD and LD at each time point for each group (treatment/placebo) was subject to linear regression to determine differences in overall trends.
Results
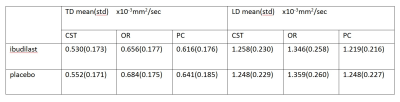
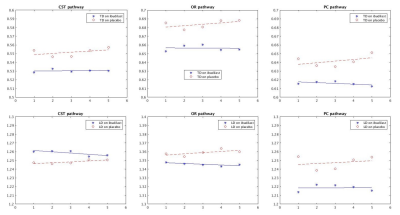
Table1 shows mean and standard deviation of TD and LD values on three pathways for patients with ibudilast and with placebo. Figure 1 shows the results of regression of the mean across subjects of TD and LD. The trend in which values stay level over time in the treatment group but change over time in the control group is preserved.Discussion and Conclusion
The results suggest that DTI on three pathways is sensitive to longitudinal changes in MS patients under placebo treatment. In conclusion, DTI may prove valuable as an imaging biomarker for longitudinal trials of MS therapy. The future analysis will examine correlation with clinical disability.Acknowledgements
We acknowledge support from the National Institute of Neurological Disorders and Stroke (U01NS082329), National Multiple Sclerosis Society (RG 4778-A-6) and from MediciNova through a contract with the National Institutes of Health.References
1. Robert J. Fox, M.D., Christopher S. Coffey, Ph.D., Robin Conwit, M.D., Merit E. Cudkowicz, M.D., Trevis Gleason, B.S., Andrew Goodman, M.D., Eric C. Klawiter, M.D., Kazuko Matsuda, M.D., Michelle McGovern, B.S., Robert T. Naismith, M.D., Akshata Ashokkumar, M.S., Janel Barnes, Ph.D., Dixie Ecklund, M.S.N., Elizabeth Klingner, M.S., Maxine Koepp, J.D., Jeffrey D. Long, Ph.D., Sneha Natarajan, Ph.D., Brenda Thornell, B.S., Jon Yankey, M.S., Robert A. Bermel, M.D., Josef P. Debbins, Ph.D., Xuemei Huang, M.S., Patricia Jagodnik, M.B.A., Mark J. Lowe, Ph.D., Kunio Nakamura, Ph.D., Sridar Narayanan, Ph.D., Ken E. Sakaie, Ph.D., Bhaskar Thoomukuntla, M.S., M.B.A., Xiaopeng Zhou, Ph.D., Stephen Krieger, M.D., Enrique Alvarez, M.D., Ph.D., Michelle Apperson, M.D., Ph.D., Khurram Bashir, M.D., Bruce A. Cohen, M.D., Patricia K. Coyle, M.D., Silvia Delgado, M.D., L. Dana Dewitt, M.D., Angela Flores, M.D., Barbara S. Giesser, M.D., Myla D. Goldman, M.D., Burk Jubelt, M.D., Neil Lava, M.D., Sharon G. Lynch, M.D., Harold Moses, M.D., Daniel Ontaneda, M.D., Jai S. Perumal, M.D., Michael Racke, M.D., Pavle Repovic, M.D., Ph.D., Claire S. Riley, M.D., Christopher Severson, M.D., Shlomo Shinnar, M.D., Ph.D., Valerie Suski, D.O., Bianca Weinstock-Guttman, M.D., Vijayshree Yadav, M.D., and Aram Zabeti, M.D. for the NN102/SPRINT-MS Trial Investigators. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N. Engl. J. Med. 2018, 379:846-855.
2. https://science.nichd.nih.gov/confluence/display/nihpd/TORTOISE
3. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996, 29(3): 162-173.
4. Lowe MJ, Beall EB, Sakaie KE, Koenig KA, Stone L, Marrie RA, Phillips MD. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum. Brain Mapp. 2008, 29:818-827.