1390
A comparison of seven white matter diffusion microstructure models to study intralesional damage in Multiple Sclerosis1Translational Imaging in Neurology (ThINk) Basel, Department of Medicine and Biomedical Engineering, University Hospital Basel and University of Basel, Neurologic Clinic and Policlinic, Basel, Switzerland, 2Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 3Digital Technology and Innovation Siemens Healthineers, Princeton, NJ, United States, 4Department of Neurology, Saint-Luc University Hospital,Université Catholique de Louvain, Bruxelles, Belgium, 5Department of Neurology, Lausanne University and University Hospital, Lausanne, Switzerland, 6Translational Neuroradiology Section, Division of Neuroimmunology and Neurovirology, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 7Signal Processing Laboratory (LTSS), Ecole polytechnique fédérale de Lausanne (EPFL), Lausanne, Switzerland, 8Radiology Department, Center for Biomedical Imaging, Lausanne University and University Hospital, Lausanne, Switzerland, Basel, Switzerland, 9Radiology Department, Center for Biomedical Imaging, Lausanne University and University Hospital Lausanne, Lausanne, Switzerland, 10Department of Computer Science, University of Verona, Verona, Italy, 11Radiological Physics, Department of Radiology, University Hospital Basel, Basel, Switzerland
Synopsis
In this study, we implemented seven microstructure models based on diffusion-weighted MRI data and investigated their sensitivity in assessing between-lesion differences in axonal content in multiple sclerosis lesions. The result of the analysis showed that all the models selected report lower intracellular volume fraction in white matter lesions compared to normal-appearing white matter. Few of the methods highlighted high sensitivity; however, they also show a different spectrum of ranges in terms of intracellular volume fraction. Whether one of the methods reflect a reliable assessment will be addressed in further studies using postmortem material.
Introduction
During the last 5 years, diffusion-weighted MRI (DW-MRI) microstructure modeling has been applied to quantify axonal damage in multiple sclerosis (MS) patients1-8. Many diffusion-based microstructural models have been proposed so far, including the widely used Neurite Orientation Dispersion and Density Imaging (NODDI) model9. To date, however, many diffusion-based microstructural models have been proposed, which may exhibit differential sensitivity to MS pathology. In this study, we implemented seven microstructure models based on DW-MRI data and investigated their sensitivity in assessing between-lesion differences in axonal content in MS lesions. Specifically, we analyzed the intracellular volume fraction (ICVF) on white matter (WM) lesions and compared one type of lesion (eg. lesions with a paramagnetic rim, rim + and not paramagnetic rim, rim-) – that are known to exhibit extensive axonal damage according to neuropathological studies10.Methods
In-vivo acquisitionDW-MRI data for 20 MS patients were acquired on a 3T Prisma scanner at the Universitatsspital Basel with the following parameters: resolution 1.8$$$mm$$$ isotropic, TR/TE/δ/Δ/=4500/75/19.2/36.5$$$ms$$$ and $$$b$$$-value=[0,700,1000,2000,3000]$$$\frac{s}{mm^2}$$$ with number of measurement=[12,6,20,45,66]. For anatomical reference and optimal segmentation of MS lesions, the MRI protocol also included an MP2RAGE image with resolution 1$$$mm$$$ isotropic, TR/TI1/TI2=5000/700/2500ms and a 3D FLAIR with resolution 1$$$mm$$$ isotropic, TR/TE/TI=5000/386/1800ms; and 3D EPI with resolution 0.67$$$mm$$$ isotropic, TR=64/35ms. DW-MRI data were processed with denoising11, eddy currents, susceptibility distortion and motion12.
Lesion segmentation and WM mask
WM lesions were automatically segmented using a pretrained Convolutional Neural Network (CNN) method13. Lesions segmentation were then corrected by two experts and the mask was registered to the DW-MRI space using rigid registration flirt14. Paramagnetic rim lesions were manually identified on 3D EPI unwrapped phase images. To study a WM area with a similar microstructure than the lesions, we selected a normal-appearing WM (NAWM) area corresponding to a 3-voxels region around each lesion. A total amount of 277 WM lesions were identified and 22 of them exhibited a paramagnetic rim.
Microstructure modeling
Eight different microstructure models were implemented using the DMIPY toolbox15: "ball and stick"16, "ball and 2 sticks"16, "ball and rockets"17, "NODDI-Watson"9, "NODDI-Bingham"18, "SMT-NODDI"19, "MCDMI"20. For the model were axial diffusivity was required to be fixed, the value chosen was 1.7 $$$\frac{mm^2}{s}$$$, as suggested in9. The ICVF was obtained for each model and compared across models by calculating the difference between the mean ICVF in WM lesions and in NAWM.
Results and discussion
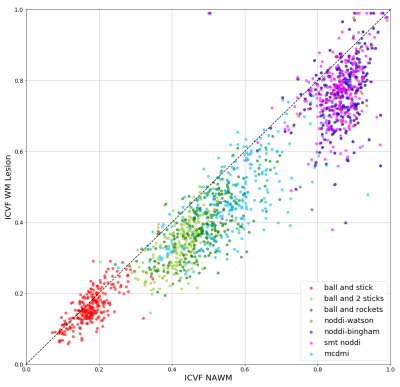
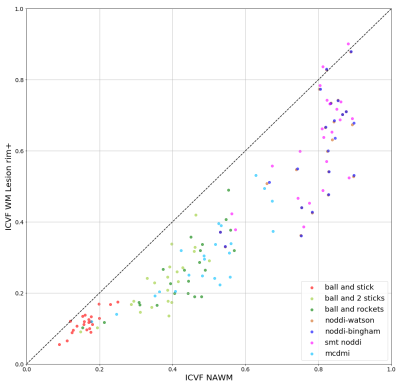
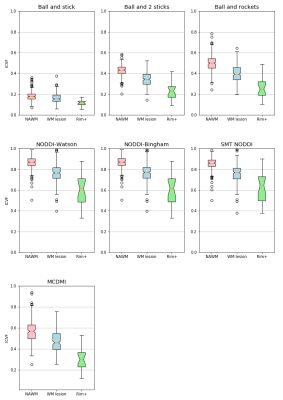
Figure 1 shows the comparison of ICVF in WM lesion and NAWM. We find that all the models report lower ICVF in the WM lesions comparing to the surrounding NAWM, as expected from the histopathology of MS lesions and surrounding normal-appearing tissue21. However, a broad spectrum of ICVF values is reported for the different models. For example, the “ball and stick” model report low ICVF both for lesion and NAWM: this finding is expected as a single stick cannot capture the complexity of the WM tissue organization. In the “ball and 2 sticks" model, the ICVF is increased as the sum of the 2 “sticks” capture better the underlying WM microstructure. The “ball and rockets” model report similar distributions compared to the “ball and 2 sticks” model. The NODDI models, “NODDI-Watson”, “NODDI-Bingham” and “SMT NODDI” report very similar results, with a high estimation of ICVF, compared to all other models: this can be explained if we consider that these models include a higher complexity compared to the previous ones because they include measures of orientation dispersion. Finally, the MCDMI model reports a much brother spectrum of ICVF compared to any previous methods.Figure 2 shows a plot of NAWM and rim+ lesions. The different models show a similar ICV spectrum to the one reported in all WM lesions. However, the ICVF rim+ values are lower compared to the ones obtained in all WM lesions (rim + and rim -). This trend is highlighted in the plot in Figure 3.
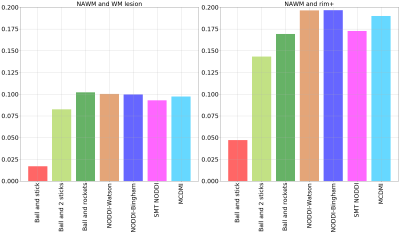
Furthermore, we investigated the sensitivity of the different models to intra-lesion pathology in MS lesions by measuring the difference between the mean ICVF in NAWM and in lesions so that the higher this difference the more sensitive is the model to MS lesion damage. Figure 4 report a comparison between NAWM and WM lesions (left) and NAWM and rim+ lesions (right). According to this estimate, the model with lowest sensitivity is the ball and stick to assess both (i) the difference in ICVF in WM lesions and NAWM and (ii) the difference in ICVF in rim + lesions and NAWM; the models with highest sensitivity to assess the difference in ICVF in all WM lesions and NAWM are the “ball and rockets”, the “NODDI-Watson” and the “NODDI-Bingham”. As to the assessment of the difference between rim + lesions and NAWM, the most performant models appeared to be NODDI-Watson” and “NODDI-Bingham”, closely followed by the“MCDMI” model.
Conclusion
We performed a comparison of seven WM microstructure models to study intralesional damage in MS patients. The most sensitive models appeared to be the “ball and rockets”, “NODDI-Watson” and the “NODDI-Bingham”, closely followed by the “MCDMI” model. Nevertheless, whether this sensitivity to ICVF changes correspond to a reliable assessment of ICVF is still an open question, which we are attempting to answer through a postmortem study.Acknowledgements
This work was funded by the Swiss National Funds PZ00P3_154508 , PZ00P3_131914 and PP00P3_176984.References
1Mallik S, Samson RS, Wheeler-Kingshott CAM, Miller DH. Imaging outcomes for trials of remyelination in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2014
2Schneider T, Brownlee W, Zhang H, Ciccarelli O, Miller DH, Wheeler-Kingshott CG, 2017. Sensitivity of multi-shell NODDI to multiple sclerosis white matter changes: A pilot study. Funct. Neurol. 2017
3By S, Xu J, Box BA, Bagnato FR, Smith SA. Application and evaluation of NODDI in the cervical spinal cord of multiple sclerosis patients. NeuroImage Clin. 2017
4Spano B, Giulietti G, Pisani V, Morreale M, Tuzzi E, Nocentini U, Francia A, Caltagirone C, Bozzali M, Cercignani M. Disruption of neurite morphology parallels MS progression. Neurol. Neuroimmunol. NeuroInflammation. 2018
5Cortese R, Collorone S, Ciccarelli O, Toosy AT. Advances in brain imaging in multiple sclerosis. Ther. Adv. Neurol. Disord. 2019
6De Santis S, Bastiani M, Droby A, Kolber P, Zipp F, Pracht E, Stoecker T, Groppa S, Roebroeck A. Characterizing Microstructural Tissue Properties in Multiple Sclerosis with Diffusion MRI at 7 T and 3 T: The Impact of the Experimental Design. Neuroscience 2019
7Hagiwara A, Kamagata K, Shimoji K, Yokoyama K, Andica C, Hori M, Fujita S, Maekawa T, Irie R, Akashi T, Wada A, Suzuki M, Abe O, Hattori N, Aoki S. White Matter Abnormalities in Multiple Sclerosis Evaluated by Quantitative Synthetic MRI, Diffusion Tensor Imaging, and Neurite Orientation Dispersion and Density Imaging. Am. J. Neuroradiol. 2019
8Mustafi S, Harezlak J, Kodiweera C, Randolph J, Ford J, Wishart H, Wu YC. Detecting white matter alterations in multiple sclerosis using advanced diffusion magnetic resonance imaging. Neural Regen. Res. 2019
9Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2010N
10Absinta M, Sati P, Reich DS. Advanced MRI and staging of multiple sclerosis lesions. Nat. Rev. Neurol. 2016
11St-Jean S, Coupé P, Descoteaux M. Non Local Spatial and Angular Matching: Enabling higher spatial resolution diffusion MRI datasets through adaptive denoising. Med. Image Anal. 2016
12Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 2003
13La Rosa F., João Fartaria M., Kober T., Richiardi J., Granziera C., Thiran J.-P., Bach Cuadra M. Shallow vs Deep Learning Architectures for White Matter Lesion Segmentation in the Early Stages of Multiple Sclerosis, MICCAI 2019
14Jenkinson M, Bannister P, Brady JM, Smith SM Improved Optimisation for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 2002
15Fick R., Wassermann D., Deriche R.. The Dmipy Toolbox: Diffusion MRI Multi-Compartment Modeling and Microstructure Recovery Made Easy. Frontiers in Neuroinformatics 2019
16Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and Propagation of Uncertainty in Diffusion-Weighted MR Imaging. Magn. Reson. Med. 2003
17Sotiropoulos SN, Behrens TEJ, Jbabdi S. Ball and rackets: Inferring fiber fanning from diffusion-weighted MRI. Neuroimage 2012
18Tariq M, Schneider T, Alexander DC, Gandini Wheeler-Kingshott CA, Zhang H. Bingham-NODDI: Mapping anisotropic orientation dispersion of neurites using diffusion MRI. Neuroimage 2016
19Cabeen RP, Sepehrband F, Toga AW Rapid and accurate NODDI parameter estimation with Spherical Mean Technique ISMRM 2019
20Kaden E, Kelm ND, Carson RP, Does MD, Alexander DC. Multi-compartment microscopic diffusion imaging. Neuroimage 2016
21Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, Mandrekar J, Bramow S, Metz I, Brück W, Lassmann H, Lucchinetti CF. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 2015
Figures