1385
Trajectories of magnetic susceptibility in the pulvinar provide further evidence for accelerated decline of thalamic iron in multiple sclerosis1Neuroimaging, Buffalo Neuroimaging Analysis Center, Buffalo, NY, United States, 2MRI Clinical and Translational Research Center, Buffalo, NY, United States, 3Neurology, Buffalo Neuroimaging Analysis Center, Buffalo, NY, United States, 4Neurology, Jacobs Comprehensive MS Center for Treatment & Research, Buffalo, NY, United States, 5Neurology, MRI Clinical and Translational Research Center, Buffalo, NY, United States
Synopsis
Several recent cross-sectional studies have observed decreasedmagnetic susceptibility in the thalamus of patients with multiple sclerosis (MS) using Quantitative Susceptibility Mapping (QSM).However, the concavity of the iron concentration trajectory in normal aging renders the interpretation of findings of the previous studies difficult. In the present work, we applied QSM longitudinally in conjunction with a dedicated analysis procedure to obtain optimal longitudinal measurement accuracy. Our longitudinal results confirm previous cross-sectional findings and suggest that thalamic QSM may serve as an imaging marker for disease progression in MS.
Synopsis
Several recent cross-sectional studies have observed decreasedmagnetic susceptibility in the thalamus of patients with multiple sclerosis (MS) using Quantitative Susceptibility Mapping (QSM).However, the concavity of the iron concentration trajectory in normal aging renders the interpretation of findings of the previous studies difficult. In the present work, we applied QSM longitudinally in conjunction with a dedicated analysis procedure to obtain optimal longitudinal measurement accuracy. Our longitudinal results confirm previous cross-sectional findings and suggest that thalamic QSM may serve as an imaging marker for disease progression in MS.Introduction
Numerous imaging studies have reported altered iron concentrations in the deep gray matter of patients with multiple sclerosis (MS). While most studies found increased iron concentrations, several recent cross-sectional studies have observed decreased magnetic susceptibility in the thalamus of patients with MS using Quantitative Susceptibility Mapping (QSM)1.A loss of oligodendroglial iron is the most plausible explanation for declining thalamic susceptibility14 rendering longitudinal thalamic QSM a promising technique for assessing oligodendroglial viability in MS. The observed strong link between pulvinar susceptibility decline and both disease duration14 and disability4 points toward the use of QSM as a maker of subclinical disease progression in MS.
However, it is well-known5 that iron in the thalamus of heathy individuals follows a peculiar non-linear trajectory that peaks between 30 and 40 years of age, and declines later in life. The concavity of this trajectory renders the interpretation of findings of the previous, cross-sectional studies difficult. In the present work, we applied QSM longitudinally in conjunction with a dedicated analysis procedure to obtain optimal longitudinal measurement accuracy.
Methods
Subjects: This retrospective study enrolled 20 patients with MS subjects and 20 age- and sex-matched controls. The average age of patients (controls) was 41.1±11.0 years (44.9±12.9 years) and female:male ratiowas 17:4 (15:5).Data acquisition: Participants underwent longitudinal MRI at 3T (GE Signa Excite HD 12.0) with a multi-channel head-neck coil utilizing a 3D GRE sequence (256x192x64 matrix, 256x192x128mm5, TE/TR=22ms/40ms, BW=13.9kHz, flip=12°). Patients were imaged a median of 5.5 times (2-12 times) over a median time of 6.7 years (5-7.5 years). Controls were imaged a median of 2.5 times (2-7 times) over a median time of 5.7 years (0.7-7.4 years).
Analysis: We reconstructed magnetic susceptibility maps from raw k-space data using scaler-phase-matching6,7, gradient unwarping8, best-path unwrapping9, LBV10-12 and HEIDI13. To minimize variation in extracted susceptibility values over time, we non-linearly warped follow-up susceptibility maps to their respective baseline maps (Python 3.0; ANTs). This procedure relied entirely on susceptibility contrast. In this study, we focused the analysis on the pulvinar nucleus of the thalamus, which demonstrated the strongest effect sizes in previous cross-sectional studies14,3. We manually segmented the pulvinar nucleus on the baseline susceptibility maps (MRIcron) and applied the labels to all co-registered follow-up scans.
Results
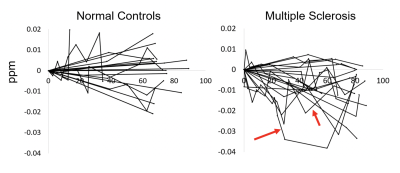
Two follow-up scans of control subjects had to be excluded from the analysis due to imaging and reconstruction artifacts, resulting in 18 patients and 20 controls with follow-up scans. Figure 1 illustrates the individual trajectories of pulvinar magnetic susceptibility in patients (right) and controls (left). 55% of the patients (16/20) but only 35% (7/20) of controls demonstrated a net decline at the last time point. While most patients demonstrated a relatively steady decline in susceptibility over time, some trajectories showed considerable variability (arrows in Figure 1).Discussion
Our data confirms that individual thalamic magnetic susceptibility values decline over timein patients with MS and that this decline occurs quicker than in controls. Systematic variations of pulvinar susceptibility over multiple follow-up time points in patients suggests that thalamic susceptibility is subject to short-term alterations potentially linked to disease activity. We plan to apply our analysis strategy to a larger cohort to further elucidate the interactions between thalamic susceptibility an age, disease duration, disability, and age at onset, and explore its use as a clinical imaging marker for disease progression.Conclusion
QSM allows assessing the longitudinal evolution of thalamic magnetic susceptibility in individual subjects. Our longitudinal results confirm previous cross-sectional findings of decreased thalamic susceptibility in MS, indicative of a disturbed oligodendroglial iron homeostasis. Findings suggest that thalamic QSM may serve as an imaging marker for disease progression in MS.Acknowledgements
No acknowledgement found.References
[1] Deistung, Andreas, et al. “Overview of Quantitative Susceptibility Mapping.” NMR in Biomedicine, vol. 30, no. 4, 2016, doi:10.1002/nbm.3569.
[2] Acosta-Cabronero, J., et al. “In Vivo MRI Mapping of Brain Iron Deposition across the Adult Lifespan.” Journal of Neuroscience, vol. 36, no. 2, 2016, pp. 364–374., doi:10.1523/jneurosci.1907-15.2016.
[3] Hagemeier, Jesper, et al. “Iron Content of the Pulvinar Nucleus of the Thalamus Is Increased in Adolescent Multiple Sclerosis.” Multiple Sclerosis Journal, vol. 19, no. 5, 2012, pp. 567–576., doi:10.1177/1352458512459289.
[4] Zivadinov, Robert, et al. “Brain Iron at Quantitative MRI Is Associated with Disability in Multiple Sclerosis.” Radiology, vol. 289, no. 2, 17 July 2018, pp. 487–496., doi:10.1148/radiol.2018180136.
[5] C. Liu, W. Li, K. A. Tong, K. W. Yeom, and S. Kuzminski, “Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain,” J Magn Reson Imaging, 42(1):23-41, 2015.
[6] J. A. de Zwart, P. J. Ledden, P. Kellman, P. van Gelderen, and J. H. Duyn, “Design of a SENSE-optimized high-sensitivity MRI receive coil for brain imaging.” Magn Reson Med, 47(6):1218–27, 2002.
[7] K. E. Hammond, J. M. Lupo, D. Xu, M. Metcalf, D. A. C. Kelley, D. Pelletier, S. M. Chang, P. Mukherjee, D. B. Vigneron, and S. J. Nelson, “Development of a robust method for generating 7.0 T multichannel phase images of the brain with application to normal volunteers and patients with neurological diseases.” NeuroImage, 39(4):1682–1692, 2008.
[8] P. Polak, R. Zivadinov, and F. Schweser, “Gradient Unwarping for Phase Imaging Reconstruction,” ISMRM 2015, p1279.
[9] H. S. Abdul-Rahman, M. A. Gdeisat, D. R. Burton, M. J. Lalor, F. Lilley, and C. J. Moore, “Fast and robust three-dimensional best path phase unwrapping algorithm.” Appl Opt, 46(26):6623–35, 2007.
[10] F. Schweser, A. Deistung, B. W. Lehr, and J. R. Reichenbach, “Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: An approach to in vivo brain iron metabolism?” NeuroImage, 54(4):2789–2807, 2011.
[11] P. S. Özbay, A. Deistung, X. Feng, D. Nanz, J. R. Reichenbach, and F. Schweser, “A comprehensive numerical analysis of background phase correction with V-SHARP,” NMR Biomed (epub)
[12] B. Wu, W. Li, A. Guidon, and C. Liu, “Whole brain susceptibility mapping using compressed sensing.” Magn Reson Med, 24:1129–36, 2011.
[13] F. Schweser, K. Sommer, A. Deistung, and J. R. Reichenbach, “Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain.” NeuroImage, 62(3):2083–2100, 2012.
[14] Schweser F, Raffaini Duarte Martins AL, Hagemeier J, et al. Mapping of thalamic magnetic susceptibility in multiple sclerosis indicates decreasing iron with disease duration: A proposed mechanistic relationship between inflammation and oligodendrocyte vitality. NeuroImage. 2018;167:438-452. doi:10.1016/j.neuroimage.2017.10.063