1378
Brainstem monoaminergic functional and structural connectivity is altered in multiple sclerosis and contributes to fatigue1Department of Neuroscience, Brighton and Sussex Medical School, University of Sussex, Brighton, United Kingdom, 2Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy, 3NeuroPoly Lab, Polytechnique Montreal, Montreal, QC, Canada, 4CUBRIC, Cardiff University, Cardiff, United Kingdom, 5School of Psychology, University of Sussex, Brighton, United Kingdom, 6Psychiatry, Psychology & Neuroscience, King’s College London, London, United Kingdom, 7Department of Psychology and Department of Medicine, Cardiff University, Cardiff, United Kingdom
Synopsis
Brainstem monoaminergic functional and structural connectivity was investigated in a cohort of patients with multiple sclerosis (MS) and healthy controls. RS-fMRI analysis revealed in MS patients reduced functional connectivity between monoaminergic nuclei and central brain networks that are critically involved in the pathophysiology of MS. Functional alterations were associated to structural disconnections between these nuclei and cortical/subcortical efferent targets in MS patients. Axonal loss in the mesocorticolimbic tracts and in the noradrenergic projections to prefrontal cortex was associated with central fatigue in MS patients, whereas brainstem functional connectivity did not correlate with fatigue.
Introduction
Brainstem monoaminergic nuclei (BrMn) contain neurons that project to the whole central nervous system, crucially modulating brain function. BrMn are directly affected in multiple sclerosis (MS) by inflammation and neurodegeneration1. Moreover, inflammation reduces the synthesis of monoamines1. Alterations in monoaminergic system may explain some of the clinical features of MS, such as fatigue2. Emerging evidence supports the role of aberrant monoaminergic neurotransmission in the pathophysiology of MS fatigue3. We focused on ventral tegmental area (VTA), locus coeruleus (LC), and median and dorsal raphe nuclei, as the main source of dopaminergic, noradrenergic, and serotoninergic neurons within the brainstem, respectively. Resting-state functional magnetic resonance imaging (RS-fMRI) and diffusion MRI were used to investigate alterations in BrMn functional and structural connectivity (Fc and Sc) in MS patients. Correlations of both BrMn Fc and Sc with MS fatigue were also evaluated.Methods
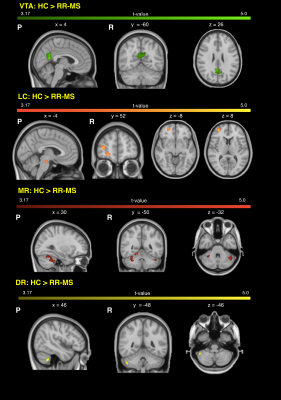
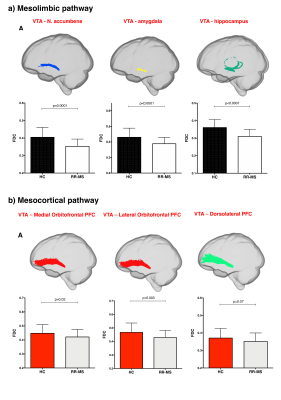
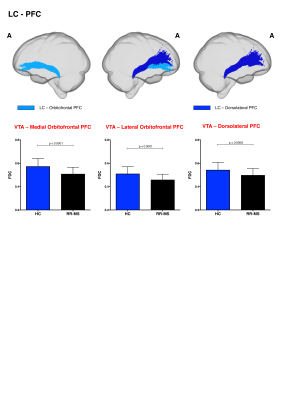
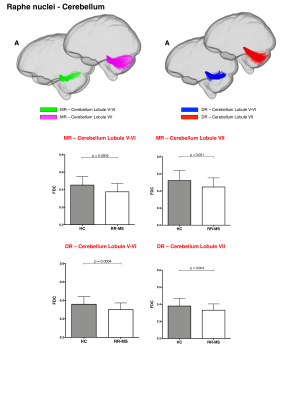
68 relapsing-remitting MS patients and 39 HC were scanned with a Siemens-Avanto 1.5T. The protocol included a T2*-weighted multi-echo echo‐planar imaging (EPI) sequence for RS-fMRI4, and a 2-shell diffusion-weighted pulsed-gradient spin-echo EPI sequence. The multi-echo sequence was chosen to minimise motion artefacts and the need for smoothing, given the small size of BrMn. BrMn Fc with the rest of the brain were evaluated in MS patients and HC using a seed-based analysis by means of SPM12, as recently described5–7. Between-group comparisons were carried out only within the main effect of HC, setting p<0.05 family-wise-error-corrected (FWEc, Fig.1). BrMn Scwas assessed using fixel-based analysis (FBA) thatallows the contemporary investigation of microscopic and macroscopic changes in white matter (WM) tracts, by calculating changes in within-voxel fibre density and fibre bundle’s cross-section (FDC)8. A tract of interest analysis was performed, considering WM bundles projecting from BrMn that resulted functional connected in the RS-fMRI analysis and represent known anatomical connections. We reconstructed the dopaminergic mesolimbic and mesocortical tracts9 (Fig.2), the projections from LC to prefrontal cortex10 (PFC; Fig.3), and the WM tracts connecting raphe nuclei to cerebellar cortex11 (Fig.4). Average FDC was calculated in each tract and compared in MS patients and HC using MRtrix312. The contribution of WM macroscopic lesion load (WM-LL) and regional GM atrophy to connectivity dysregulation was also investigated, by performing WM lesions probability maps and voxel-based morphometry (VBM) with SPM12, respectively. MS patients were divided according to fatigue status, using the modified fatigue impact scale13 (MFIS) and correlations of Fc and Sc with MFIS scores were tested using the Spearman rank correlation coefficient. For every analysis, statistical significance was set to p<0.05, except for the FBA in which it was set to p<0.004, after Bonferroni correction.Results
VTA in HC resulted functionally connected with the core regions of the default-mode network6. As compared to HC, MS patients displayed altered Fc between VTA and posterior cingulate cortex (p<0.05FWEc; Fig.1). A reduced FDC in MS patients, as compared to HC, was found in MS patients in all WM tracts of the mesolimbic pathway (p<0.0001), but not in the mesocortical tracts (Fig.2). LC displayed positive Fc with core regions of the executive-control network with a reduced functional connection between LC and right PFC in MS patients (p<0.05FWEc; Fig.1). LC projections to PFC displayed structural WM damage in MS patients (p<0.0001; Fig.3). Raphe nuclei resulted functionally connected with cerebellar cortex, with a significant lower Fc between these nuclei and cerebellum in MS patients, as compared to HC (p<0.05FWEc; Fig.1). All WM tracts projecting from raphe nuclei to cerebellar cortex displayed lower FDC in MS patients (p=0.0002, p=0.0004, respectively; Fig.4). No correlation between BrMCn Fc and fatigue was observed. Conversely, a modest association between BrMCn Sc and cognitive fatigue was found in MS patients. Particularly, we observed significant correlations between the MFIS cognitive subscale and the FDC within all the WM tracts projecting from VTA (VTA-nucleus accumbens r=-0.29; p=0.02; VTA-amygdala r=-0.28; p=0.01; VTA-hippocampus r=-0.36; p=0.002; VTA-dorsolateral-PFC r=-0.25; p=0.03; VTA-orbitofrontal-PFC r=-0.30; p=0.01), and from LC to PFC (LC-dorsolateral-PFC r=-0.29; p=0.01; LC-orbitofrontal-PFC r=-0.28; p=0.01). Loss of FDC within the WM tracts projecting from raphe nuclei did not correlate with fatigue. WM-LL frequency in the brainstem was observed to be less than 2%. VBM analyses revealed significant GM loss in MS patients only in bilateral thalamus (p<0.0001FWEc), as compared to HC.Discussion
Our findings confirmed that BrMn are functionally integrated within central brain networks involved in MS pathology and showed that these connections are altered in MS patients. We demonstrated in MS patients a structural disconnection between BrMn and cortical/subcortical efferent targets, due to selective WM loss along specific fibre tracts projecting from BrMn. These structural alterations may – at least partially – explain the functional alterations, while GM atrophy and macroscopic WM lesion load did not seem to influence them. Finally our study supported the hypothesis of a contribution of the dopaminergic mesocorticolimbic pathway to central fatigue in MS, and suggest a potential involvement of the noradrenergic LC-PFC pathway, possibly linked to axonal loss that imbalances monoaminergic neurotransmission.Conclusions
We used multimodal MRI techniques to obtain a comprehensive analysis of BrMn Fc and Sc in MS, finding a dysregulation of brainstem monoaminergic connectivity in MS. These findings add new information about how monoaminergic system contributes to MS pathogenesisand suggest new therapeutic targets.Acknowledgements
MM was funded by the Wellcome Trust through a Sir Henry Wellcome Postdoctoral Fellowship.References
1. Dobryakova, E., Genova, H. M., DeLuca, J. & Wylie, G. R. The Dopamine Imbalance Hypothesis of Fatigue in Multiple Sclerosis and Other Neurological Disorders. Front. Neurol.6, (2015).
2. Genova, H. M. et al.Examination of Cognitive Fatigue in Multiple Sclerosis using Functional Magnetic Resonance Imaging and Diffusion Tensor Imaging. PLoS ONE8, e78811 (2013).
3. Manjaly, Z.-M. et al.Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry90, 642–651 (2019).
4. Poser, B. A., Versluis, M. J., Hoogduin, J. M. & Norris, D. G. BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: parallel-acquired inhomogeneity-desensitized fMRI. Magn Reson Med55, 1227–1235 (2006).
5. Kundu, P. et al.Multi-echo fMRI: A review of applications in fMRI denoising and analysis of BOLD signals. NeuroImage154, 59–80 (2017).
6. Bär, K.-J. et al.Functional connectivity and network analysis of midbrain and brainstem nuclei. NeuroImage134, 53–63 (2016).
7. Serra, L. et al.In vivo mapping of brainstem nuclei functional connectivity disruption in Alzheimer’s disease. Neurobiology of Aging72, 72–82 (2018).
8. Raffelt, D. A. et al.Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage144, 58–73 (2017).
9. Coenen, V. A. et al.The anatomy of the human medial forebrain bundle: Ventral tegmental area connections to reward-associated subcortical and frontal lobe regions. NeuroImage: Clinical18, 770–783 (2018).
10. Kline, R. L. et al.The Effects of Methylphenidate on Resting-State Functional Connectivity of the Basal Nucleus of Meynert, Locus Coeruleus, and Ventral Tegmental Area in Healthy Adults. Front. Hum. Neurosci.10, (2016).
11. Oostland, M. & van Hooft, J. A. Serotonin in the Cerebellum. in Essentials of Cerebellum and Cerebellar Disorders(eds. Gruol, D. L. et al.) 243–247 (Springer International Publishing, 2016). doi:10.1007/978-3-319-24551-5_31
12. Tournier, J.-D. et al.MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage202, 116137 (2019).
13. Fisk, J. D. et al.Measuring the Functional Impact of Fatigue: Initial Validation of the Fatigue Impact Scale. Clinical Infectious Diseases18, S79–S83 (1994).
Figures