1376
Declining frontoparietal connectivity is linked to decreased episodic memory performance in multiple sclerosis1Imaging Sciences, The Cleveland Clinic, Cleveland, OH, United States, 2Neurological Institute, The Cleveland Clinic, Cleveland, OH, United States, 3Schey Center for Cognitive Neuroimaging, The Cleveland Clinic, Cleveland, OH, United States
Synopsis
Cognitive dysfunction, often including memory loss, impacts about half of those with Multiple Sclerosis (MS). Our work aims to develop a predictor of future memory decline in MS. Using high resolution MRI, we measured resting state functional connectivity of the frontoparietal network in 77 participants with MS. We found that connectivity was related to episodic memory at baseline, and that the one-year change in connectivity was related to the change in memory performance. This finding suggests that functional connectivity can be developed as a predictor of memory decline in MS.
Introduction
Cognitive decline is a common symptom of Multiple Sclerosis (MS), affecting about half of patients.1 Patients often experience memory loss, which can have a major impact on quality of life. A measure that can predict which patients are at risk of cognitive decline would give patients and their physicians important information for treatment decisions and future planning. Our previous work found that resting state functional connectivity (rs-fMRI) within the frontoparietal network was related to cognitive performance in MS, particularly to episodic memory.2 Here we confirm this finding and report a longitudinal relationship between rs-fMRI and memory.Methods
In an IRB-approved protocol, 77 patients with MS [mean age: 51.45 ± 8.3, 19 males, mean EDSS: 4.0 ± 1.6] were scanned on a Siemens 7T Magnetom with a SC72 gradient (Siemens Medical Solutions, Erlangen) using a 32-channel head coil (Nova Medical). Participants also completed a measure of verbal episodic memory, the Selective Reminding Test (SRT). After one year, fifty participants returned for repeat scanning and cognitive testing using counterbalanced, equivalent measures. Change in SRT score was calculated as the subtraction of the normalized scores (visit 2 – visit 1).MRI acquisition
A whole-brain anatomical MP2RAGE (0.75mm isotropic voxel size) and an rs-fMRI scan were acquired. Rs-fMRI acquisition parameters were: 132 repetitions of 81 1.5mm thick axial slices acquired with TE/TR=21ms/2800ms, matrix 160x160, FOV 210mm x 210mm, receive bandwidth = 1562 Hz/pixel. Subjects were instructed to keep their eyes closed during scans.
Data analysis
Rs-fMRI scans were corrected for motion and physiologic noise, detrended, and lowpass filtered.3,4 For individual baseline scans, a previously described method and a functionally-defined template5,6 were used to define 9-voxel in-plane seeds in the left dorsal lateral prefrontal cortex (DLPFC). Baseline seeds were co-registered to follow-up scans. Seeds were used to calculate whole-brain correlation maps, which were normalized7 and transformed to common space using non-linear warping. Baseline rs-fMRI maps were averaged, and the thresholded average map was used to produce a mask of voxels within the frontoparietal network. For baseline participants, SRT score for each participant was correlated with connectivity for each voxel included in the mask, producing a voxel-wise map of the strength of correlation between rs-fMRI and SRT within the frontoparietal network. For significant regions, change in connectivity was calculated as visit 2 – visit 1 and correlated with SRT measures.
Results
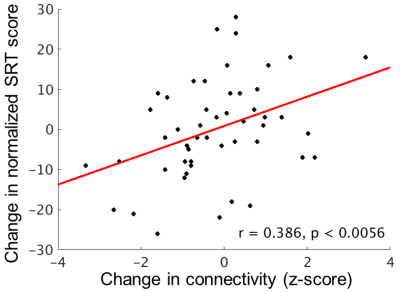
Figure 1 shows average connectivity to the left DLPFC (p < 1x10-6, cluster size 500). At baseline, the strength of connectivity from the left DLPFC to the right middle frontal gyrus (MFG, BA46; outlined in green in Figure 1) and to the left inferior parietal lobule (IPL, BA40; outlined in blue in Figure 1) was significantly related to SRT score (p < 0.01, cluster size = 125).Baseline connectivity to the right MFG was related to SRT score at follow-up (r = 0.359, p < 0.010). The change in connectivity to the left IPL was related to the change in SRT score (r = 0.386, p < 0.0056; Figure 2), so that those with a decline in connectivity strength were more likely to show a decline in memory score.
Discussion
Our baseline sample confirms our previous finding that rs-fMRI within the frontoparietal network is related to memory performance. Our investigation of the frontoparietal network stems from previous reports linking it to overall cognitive ability. While our primary focus is memory dysfunction, the frontoparietal network has been conceptualized as a functional hub, modulating and coordinating other networks.8 It may be that episodic memory is particularly sensitive to dysfunction of this network. The results of the longitudinal analysis suggest that investigation of frontoparietal connectivity as a predictor of memory decline in MS is well-founded. Future work will include additional collection of longitudinal data and development of prediction models.Acknowledgements
This work was supported by the Department of Defense (MS150097). We thank Siemens Healthineers Tobias Kober for use of WIP944 and Thomas Benner for use of WIP770B.References
1. Achiron A, Chapman J, Magalashvili D, Dolev M, Lavie M, Bercovich E, et al. Modeling of Cognitive Impairment by Disease Duration in Multiple Sclerosis: A Cross-Sectional Study. PLoS One. 2013;8(8):e71058
2. Koenig, K.A., Sakaie, K.E., Lowe, M.J., Lin, J., Stone, L., Bermel, R.A., Beall, E.B., Rao, S.M., Trapp, B.D., Phillips, M.D. (2013, June) Dorsolateral prefrontal connectivity relates to episodic memory in Multiple Sclerosis. Poster presented at the meeting of the Organization for Human Brain Mapping, Seattle, Washington.
3. Beall EB and Lowe MJ. (2014) SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage. 101:21-34.
4. Glover et al. (2000) Image-Based Method for Retrospective Correction of Physiological Motion Effects in fMRI: RETROICOR. Magnetic Resonance in Medicine. 44:162-67.
5. Sallet J, Mars RB, Noonan MP, et al. (2013) The Organization of Dorsal Frontal Cortex in Humans and Macaques. The Journal of Neuroscience. 33: 12255-74
6. Lowe et al. (2014) Anatomic connectivity assessed using pathway radial diffusivity is related to functional connectivity in monosynaptic pathways. Brain Connectivity. 4(7):558-65.
7. Lowe et al. (1998) Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 7(2):119-32.
8. Marek et al. (2018) The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neuroscience. 20(2):133-140.
Figures