1366
Cross-cortical Depth-dependent Interactions in the Human Brain using EPIK1Forschungszentrum Jülich - INM-4, Jülich, Germany, 2Forschungszentrum Jülich - INM-11, Jülich, Germany, 3JARA-BRAIN, Aachen, Germany, 4RWTH Aachen University, Aachen, Germany
Synopsis
Cross-cortical interactions in the human brain remain poorly understood and are often over-simplified as a 2-dimensional cortical model in fMRI studies. To date, high-resolution fMRI has been limited to relatively small brain slabs that cover particular areas of interest, providing fine mapping of local circuits but precluding macroscale analysis. Here, an EPIK sequence was used to measure the GE-BOLD signal from individual cortical layers through most of the brain. The combination of high resolution (0.63 mm isotropic) and large coverage fMRI enabled identification of long-distance neuronal interactions that take place between particular cortical depths during resting-state.
Introduction
The neocortex in the mammalian brain is organized, in depth, into 6 well-defined histological layers. The way in which the cortical neurons communicate with each other in the human brain (across different depths and through different areas) remains largely unknown, primarily due to a lack of non-invasive methods to sufficiently sample the cortical mantle. However, recently, layer-based functional MRI (fMRI) has enabled the investigation of inter-laminar behaviour within defined regions engaged in particular tasks.1-3 Despite this advance, the limited brain coverage of the existing high-resolution-fMRI schemes precludes the investigation of large-scale neuronal cross-talk in the human cortex. It has been previously shown that EPIK can offer higher resolution with comparable performance in identifying functional signals.4-9 Therefore, in order to achieve high-resolution fMRI with near-whole-brain coverage, here an EPIK-based sequence10-13 was optimised to provide submillimetre isotropic resolution (0.63 mm3) with 123 slices to acquire resting-state fMRI in a healthy volunteer. A novel layer-based analysis approach enabled detection of depth-dependent connectivity on a macro-scale.Methods
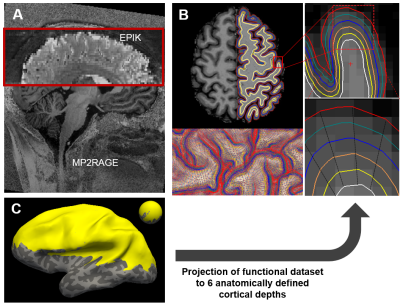
In order to investigate potentially different connectivity patterns across individual cortical layers, a resting-state paradigm was employed, which enhances the spontaneous synchronous firing of distant neuronal populations (i.e. resting-state networks). This brain state differs from that induced during evoked fMRI studies, where the activation area is well localised and, hence, a large field-of-view is often not required. In contrast, resting-state fluctuations occur broadly and, therefore, whole-brain coverage is a prerequisite for characterisation. Here, an EPIK sequence was employed to acquire GE-BOLD images with 0.63 mm isotropic resolution and near whole-brain coverage (Fig. 1A&C). The achieved voxel size is comparable with that used in current layer-fMRI studies, but the matrix size is significantly larger than the one reported in the literature, which indicates that the proposed method covers a much larger FOV. In total, 206 volumes were acquired in a 7T scanner (Siemens Magnetom Terra) using the following parameters: TR/TE: 3500/22 ms, FOV = 210 × 210 mm2, matrix = 336 × 336 × 123 slices (0.625 × 0.625 × 0.63 mm3), partial Fourier = 5/8 and 3-fold in-plane/3-fold inter-plane (multi-band) acceleration. Additionally, MP2RAGE data were acquired to perform brain segmentation and to generate six surfaces between the outer and the inner limit of the cortex (Fig. 1B) using FreeSurfer. Pre-processing of the fMRI data was performed with SPM and AFNI, which included the following steps: slice timing correction, realignment and regression of 12 motion parameters in the magnitude-reconstructed images. Additionally, the phase data of the fMRI scans, having undergone the same pre-processing steps, were unwrapped in the temporal domain and regressed out from the magnitude fMRI scans to partially correct for large-vein contamination.14,15 The corrected fMRI scans were then mapped to the 6 MP2RAGE-derived surfaces. Seven ROIs were manually drawn, based on the results of an independent component analysis (FSL), and the time courses of individual vertices were averaged together per surface and per ROI. Power spectrum analysis was computed to identify activity changes through the different layers across the cortex. Additionally, eigenvector centrality mapping16 was computed per surface, and a connectivity matrix was generated including 84 clusters (7 ROIs × 6 surfaces × 2 cerebral hemispheres).Results
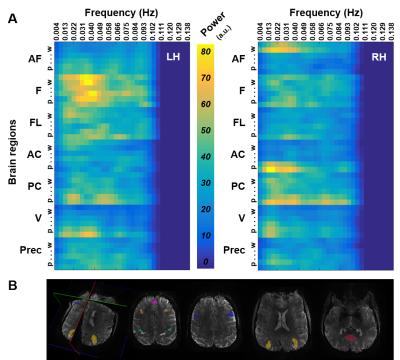
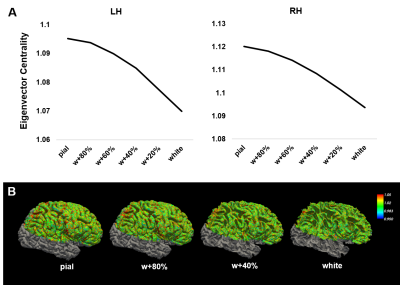
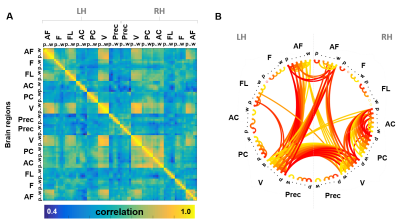
Spectral analysis of the signals acquired from different cortical depths across distant regions revealed distinct activation patterns through both cortical hemispheres (Fig. 2), with most ROIs exhibiting their maximum power in the upper layers for nearly all frequency bands studied. In agreement with this, the outer surface of the cortex presented a greater degree of centrality when compared to deeper layers (Fig. 3). High-degree correlation analysis identified particular connections between clusters located at different depths and regions, especially, but not limited to, the superficial layers (Fig. 4A&B), thus, further demonstrating the heterogeneous behaviour of the cortex. Graph analysis identified reliable intra- and inter-hemispheric connectivity patterns, especially between the anterior frontal cortex and the visual cortex (Fig. 4B), which verifies the capabilities of high-resolution EPIK for whole-brain mapping.Discussion / conclusions
The presented results indicate evidence of a depth-dependent orchestration of neuronal activity in the resting human brain. An optimised version of EPIK enabled expansion of the boundaries of current high-resolution fMRI to infer inter-laminae connectivity through the whole cortex, adding a further dimension to the current resting-state fMRI. Analysis of the data remains challenging due to the high computational demands and the difficulty of achieving perfect whole-brain co-registration between the functional and the anatomical images. However, taken together, our results demonstrate the potential of the EPIK method to track neuronal oscillations with layer specificity through the whole-brain.Acknowledgements
We thank Elke Bechholz for technical support and the fMRI volunteers for their excellent cooperation.References
1. Huber L, Handwerker DA, Jangraw DC, et al. High-Resolution CBV-fMRI Allows Mapping of Laminar Activity and Connectivity of Cortical Input and Output in Human M1. Neuron, 2017. 96(6):1253-1263.
2. Kok P. Bains LJ, van Mourik T, et al. Selective Activation of the Deep Layers of the Human Primary Visual Cortex by Top-Down Feedback. Curr Biol, 2016. 26(3):371-376.
3. Kay K, Jamison KW, Vizioli L, et al. A critical assessment of data quality and venous effects in sub-millimeter fMRI. Neuroimage, 2019. 189:847-869.
4. Yun SD, Reske M, Vahedipour K, et al. Parallel imaging acceleration of EPIK for reduced image distortions in fMRI. Neuroimage, 2013. 73:135-143.
5. Yun SD and Shah NJ. Whole-brain high in-plane resolution fMRI using accelerated EPIK for enhanced characterisation of functional areas at 3T. PLoS One, 2017. 12(9):e0184759.
6. Caldeira LL, Yun S, da Silva NA, et al. Dynamic susceptibility contrast parametric imaging using accelerated dual-contrast echo planar imaging with keyhole. J Magn Reson Imaging. 2019; 50(2):628-640.
7. Shah NJ, da Silva NA, and Yun S. Perfusion weighted imaging using combined gradient/spin echo EPIK: Brain tumour applications in hybrid MR-PET. Hum Brain Mapp. 2019 Feb 13.
8. Yun SD, Weidner R, Weiss PH, et al. Evaluating the Utility of EPIK in a Finger Tapping fMRI Experiment using BOLD Detection and Effective Connectivity. Sci Rep, 2019. 9(1):10978.
9. Yun S, and Shah NJ. Full-FOV, Whole-brain, Half-millimetre Resolution fMRI at 7T using Accelerated multi-band EPIK with TR-external Phase Correction. In Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada, 2019. Abstract 1167.
10. Shah NJ and Zilles K. Verfahren zur Untersuchung eines Objektes mittels Erfassung des Ortsfrequenzraumes. 2003. German Patent Application No. 199 62 845 C2.
11. Shah NJ, and Zilles K. Imaging process in the spatial frequency space and useful for examining the properties of object. 2004. USA Patent Application No. 6781372 B2.
12. Zaitsev M, Zilles K, and Shah NJ. Shared k-space echo planar imaging with keyhole. Magn Reson Med. 2001; 45(1):109-117.
13. Zaitsev M, D'Arcy J, Collins DJ, et al. Dual-contrast echo planar imaging with keyhole: application to dynamic contrast-enhanced perfusion studies. Phys Med Biol. 2005; 50(19):4491-4505.
14. Menon RS. Postacquisition suppression of large-vessel BOLD signals in high-resolution fMRI. Magn Reson Med, 2002. 47(1):1-9.
15. Curtis AT, Hutchison RM, and Menon RS. Phase based venous suppression in resting-state BOLD GE-fMRI. Neuroimage, 2014. 100:51-59.
16. Lohmann G, Margulies DS, Horstmann A, et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS One, 2010. 5(4):e10232.
Figures