1353
Functional MRI investigation of Optogenetically-evoked Spindle-like Neural Activity and Memory Consolidation
Xunda Wang1,2, Alex T. L. Leong1,2, Shawn Zheng kai Tan3, Teng Ma1,2, Pek-Lan Khong4, Lee-Wei Lim3, and Ed Xuekui Wu1,2,3,4
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, Hong Kong, 3School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong, 4Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, Hong Kong, 3School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong, 4Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong
Synopsis
Spindle is one of the most critical brain oscillatory activities that has been shown to mediate sensory transmission and memory consolidation. However, where and how spindle-related activities are distributed at the systems level and their brain-wide propagation targets remain elusive. In this study, we demonstrate the first integrative view of the causal recruitment of brain-wide networks by thalamo-cortically initiated spindle-related activities in a temporal-frequency specific manner and verified its role in facilitating memory consolidation.
Purpose
Brain-wide spatiotemporal patterns arise from coordinated interactions between large groups of neurons distributed across different circuits and systems1-4. Spindle is one of the most critical oscillatory activities that has been shown to orchestrate thalamic, cortical and hippocampal neuronal populations to mediate sensory transmission and memory consolidation5-9. They are 0.5-3s, 7-14Hz brief brain oscillatory events that are presently attributed to originate from localized interactions in thalamo-cortical circuits. Considerable efforts have been devoted in an attempt to document spindle activity and their propagation characteristics using electroencephalography (EEG) topography9-15 or EEG activity-triggered blood-oxygenation-level-dependent functional MRI (BOLD-fMRI) mapping16-22. However, these studies rely on EEG recordings that have limited density and only represent cortical surface activities without incorporating neural activities at deep brain and subcortical regions. Consequently, they are insufficient to provide comprehensive insights about the extent of brain-wide spindle-related activities. In particular, where and how brain-wide spindle-related activities are distributed and their propagation targets remain unknown and poorly understood. To address these questions, we combine spatiotemporal and cell-type-specific optogenetic stimulation with fMRI to simultaneously evoke thalamo-cortical spindle-like activities that mimic localized spontaneous spindle events and monitor their brain-wide responses and propagation characteristics. As an additional exploratory goal, we also examine the functional relevance of such brain-wide spindle-related activities to memory consolidation through visual fMRI and behavior experiments.Method
Animal preparation and MRI experimental setup: 3μl AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to ventral posteromedial thalamus (VPM) of SD rats. After 4 weeks (Figure 1A), an opaque optical fiber cannula was implanted at VPM. All fMRI experiments were performed under 1.0% isoflurane. MRI data were acquired at 7T using GE-EPI.Optogenetic fMRI and electrophysiology experiments: 8, 16, 24 and 96 pulses 8Hz or 24 pulses 4, 14, and 20Hz of blue (473nm) light optogenetic stimulations were presented every 30s (10ms pulse width, 40mW/mm2; Figure 1B). Significant BOLD responses was identified by coherence analysis23. BOLD signal profiles were extracted from atlas-based ROIs. To verify whether the brain-wide BOLD responses were evoked by spindle-like neural activities, electrophysiology data (Figure 3A) was acquired using a multichannel electrode (16 channels) at primary somatosensory barrel field (S1BF) and 5 single electrodes at VPM, amygdala (Amg), retrosplenial (RS), prelimbic cortex (PrL) and superior colliculus (SC).
Visual fMRI and behavior experiments: Visual fMRI/fear conditioning experiments were conducted before and after 40 mins of 24 pulses 8Hz spindle-like stimulation (Figure 4A and 5A). 10s 5Hz visual stimulation was employed for visual fMRI and as the conditioned stimulus (CS) for fear conditioning experiments. Visual responses were analyzed via a GLM model and ROI-based BOLD characterization. Freezing rates were examined for each 5 blocks of CS during extinction phase of the behavior experiments (Figure 5B).
Results
Temporal characteristics dictate brain-wide coordination of spindle-like neural activities: Optogenetic stimulations of VPM at the typical temporal-frequency range of spindles (i.e., 8-24 pulses at 8-14Hz) evoked robust brain-wide BOLD activations (Figure 2A and B) that are more widespread than stimulations outside the range (i.e., 96 pulses 8Hz or 24 pulses 4/20Hz). Strongest brain-wide spindle-related activations (i.e., 24 pulses 8Hz stimulation) included the sensorimotor cortices, thalamus and midbrain regions (somatosensory, visual, etc.), higher-order sensory and motor-related cortices (insula, piriform and parietal), limbic regions (amygdala, hippocampus and cingulate, etc.), and basal-ganglia (caudate putamen and substantia nigra). In general, cortical responses were widespread, while subcortical responses were confined to the ipsilateral hemisphere (Figure 2A, B and D). The electrophysiology results confirmed that the optogenetically-evoked spindle-like thalamo-cortical activities follow typical features of spindles in frequency, duration, waxing-and–waning shape of the neural activity’s amplitude envelops, and slow oscillation-spindle coupling (Figure 3).Optogenetically-evoked brain-wide spindle-like activities enhance consolidation of visual memory: We found that after (post) optogenetic spindle-like stimulation, visual responses increased significantly in the visual pathway (e.g., SC). Moreover, we also found that numerous higher-order regions, such as Amg, Cg, PrL, RS, and orbital frontal cortex, were activated post-spindle-like stimulation (Figure 4B and C). These observations were not found in sham animals. Furthermore, freezing rates of OG animals during extinction phase were elevated significantly longer than those of sham and naïve animals (Figure 5B).
Discussion
Our results demonstrated that the optogenetic stimulation of VPM, specifically within the range of typical spindle duration (<3s) and frequency (8-14Hz), evokes robust brain-wide BOLD response including sensorimotor-related thalamo-cortical and midbrain regions, limbic system, and basal-ganglia. This brain-wide spindle-related BOLD activation pattern propagated the furthest at 24 pulses 8Hz stimulation, recruiting multiple deep and small brain regions (e.g., amygdala and entorhinal areas) that had only been implicated separately by invasive electrophysiology studies24-26. Our electrophysiology results confirmed that our evoked spindle-like activities were similar to spontaneous27,28 or optogenetically-evoked29,30 spindles. Together with the BOLD activation patterns, these results demonstrated that the strength of brain-wide spindle-related activities was dictated by temporal characteristics of the optogenetic spindle-like stimulation. Furthermore, brain-wide spindle-related activities evoked by 24 pulses 8Hz led to the enhancement of visual memory consolidation. This suggests that brain-wide spindle-related activation together with its coupled slow oscillation could facilitate brain-wide functional connectivity of critical circuits17,20,31,32 for consolidating such cross-modality memory. In conclusion, our study documents for the first time where and how spindle-like activity is distributed brain-wide to provide critical insights into spindle activity at the systems level.Acknowledgements
This study is supported in part by Hong Kong Research Grant Council (C7048-16G and HKU17115116 to E.X.W. and HKU17103819 to A.T.L.), Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001) and Guangdong Key Technologies for Alzheimer’ Disease Diagnosis and Treatment (2018B030336001) to E.X.W.References
- Buzsaki, G. Rhythms of the Brain, (Oxford University Press, 2006).
- Buzsaki, G., Logothetis, N. & Singer, W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 80, 751-764 (2013).
- Florin, E., Watanabe, M. & Logothetis, N.K. The role of sub-second neural events in spontaneous brain activity. Curr Opin Neurobiol 32, 24-30 (2015).
- Steriade, M., McCormick, D.A. & Sejnowski, T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679-685 (1993).
- Astori, S., Wimmer, R.D. & Luthi, A. Manipulating sleep spindles--expanding views on sleep, memory, and disease. Trends in Neurosciences 36, 738-748 (2013).
- Luthi, A. Sleep Spindles: Where They Come From, What They Do. Neuroscientist 20, 243-256 (2014). Antony, J.W., Schonauer, M., Staresina, B.P. & Cairney, S.A. Sleep Spindles and Memory Reprocessing. Trends Neurosci 42, 1-3 (2019).
- Staresina, B.P., Bergmann, T.O., Bonnefond, M., van der Meij, R., Jensen, O., Deuker, L., Elger, C.E., Axmacher, N. & Fell, J. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nature Neuroscience 18, 1679-1686 (2015).
- Mak-McCully, R.A., Rolland, M., Sargsyan, A., Gonzalez, C., Magnin, M., Chauvel, P., Rey, M., Bastuji, H. & Halgren, E. Coordination of cortical and thalamic activity during non-REM sleep in humans. Nature Communications 8, 15499 (2017).
- Nir, Y., Staba, R.J., Andrillon, T., Vyazovskiy, V.V., Cirelli, C., Fried, I. & Tononi, G. Regional slow waves and spindles in human sleep. Neuron 70, 153-169 (2011).
- Helfrich, R.F., Lendner, J.D., Mander, B.A., Guillen, H., Paff, M., Mnatsakanyan, L., Vadera, S., Walker, M.P., Lin, J.J. & Knight, R.T. Bidirectional prefrontal-hippocampal dynamics organize information transfer during sleep in humans. Nat Commun 10, 3572 (2019).
- Andrillon, T., Nir, Y., Staba, R.J., Ferrarelli, F., Cirelli, C., Tononi, G. & Fried, I. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci 31, 17821-17834 (2011).
- Piantoni, G., Halgren, E. & Cash, S.S. Spatiotemporal characteristics of sleep spindles depend on cortical location. Neuroimage 146, 236-245 (2017).
- Purcell, S.M., Manoach, D.S., Demanuele, C., Cade, B.E., Mariani, S., Cox, R., Panagiotaropoulou, G., Saxena, R., Pan, J.Q., Smoller, J.W., Redline, S. & Stickgold, R. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun 8, 15930 (2017).
- Dehghani, N., Cash, S.S. & Halgren, E. Topographical frequency dynamics within EEG and MEG sleep spindles. Clin Neurophysiol 122, 229-235 (2011).
- Schabus, M., Dang-Vu, T.T., Albouy, G., Balteau, E., Boly, M., Carrier, J., Darsaud, A., Degueldre, C., Desseilles, M., Gais, S., Phillips, C., Rauchs, G., Schnakers, C., Sterpenich, V., Vandewalle, G., Luxen, A. & Maquet, P. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A 104, 13164-13169 (2007).
- Boutin, A., Pinsard, B., Bore, A., Carrier, J., Fogel, S.M. & Doyon, J. Transient synchronization of hippocampo-striato-thalamo-cortical networks during sleep spindle oscillations induces motor memory consolidation. Neuroimage 169, 419-430 (2018).
- Dang-Vu, T.T. Functional neuroimaging insights into the physiology of human sleep. Sleep (2010).
- Fogel, S.M., Albouy, G., Vien, C., Popovicci, R., King, B.R., Hoge, R., Jbabdi, S., Benali, H., Karni, A., Maquet, P., Carrier, J. & Doyon, J. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum Brain Mapp 35, 3625-3645 (2014).
- Andrade, K.C., Spoormaker, V.I., Dresler, M., Wehrle, R., Holsboer, F., Samann, P.G. & Czisch, M. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci 31, 10331-10339 (2011).
- Mander, B.A., Rao, V., Lu, B., Saletin, J.M., Ancoli-Israel, S., Jagust, W.J. & Walker, M.P. Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cereb Cortex 24, 3301-3309 (2014).
- Bergmann, T.O., Molle, M., Diedrichs, J., Born, J. & Siebner, H.R. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage 59, 2733-2742 (2012).
- Lee, J.H., Durand, R., Gradinaru, V., Zhang, F., Goshen, I., Kim, D.S., Fenno, L.E., Ramakrishnan, C. & Deisseroth, K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792 (2010).
- Berke, J.D., Okatan, M., Skurski, J. & Eichenbaum, H.B. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43, 883-896 (2004).
- Lazarus, M., Chen, J.F., Urade, Y. & Huang, Z.L. Role of the basal ganglia in the control of sleep and wakefulness. Curr Opin Neurobiol 23, 780-785 (2013).
- Saunders, A., Oldenburg, I.A., Berezovskii, V.K., Johnson, C.A., Kingery, N.D., Elliott, H.L., Xie, T., Gerfen, C.R. & Sabatini, B.L. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521, 85-89 (2015).
- Coppieters 't Wallant, D., Maquet, P. & Phillips, C. Sleep Spindles as an Electrographic Element: Description and Automatic Detection Methods. Neural Plast 2016, 6783812 (2016).
- Contreras, D. & Steriade, M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. The Journal of Physiology 490, 159-179 (1996).
- Kim, A., Latchoumane, C., Lee, S., Kim, G.B., Cheong, E., Augustine, G.J. & Shin, H.S. Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proc Natl Acad Sci U S A 109, 20673-20678 (2012).
- Latchoumane, C.-F., Ngo, H.-V., Born, J. & Shin, H.-S. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424-435 (2017).
- Wang, X., Leong, A.T., Chan, R.W., Liu, Y. & Wu, E.X. Thalamic low frequency activity facilitates resting-state cortical interhemispheric MRI functional connectivity. NeuroImage 201, 115985 (2019).
- Wang, X., Leong, A.T., Audrey, G.S., Dong, C.M. & Wu, E.X. Optogenetically-evoked spindle-like activity from thalamus propagates brain-wide and enhances rsfMRI connectivity. in Proceedings of the 27th Annual Meeting of ISMRM 3752 (International Society for Magnetic Resonance in Medicine, Montreal, Canada, 2019).
Figures
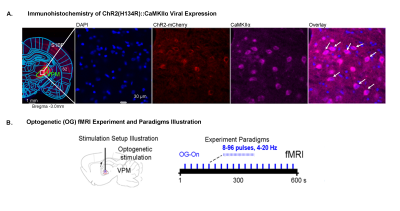
Histological characterization of viral
expression in VPM thalamocortical excitatory neurons and optogenetic fMRI
experiment setup and paradigm. (A) Confocal images of ChR2-mCherry expression in
VPM with lower and higher magnification. Overlay of images for the nuclear
marker DAPI, excitatory marker CaMKIIα, and mCherry revealed colocalization of
mCherry and CaMKIIα in thalamo-cortical neurons (white arrows). (B)
Illustration of optogenetic stimulation setup and experimental timeline (left)
with different optogenetic stimulation paradigms for a typical OG-fMRI scan.

Brain-wide BOLD activations upon different durations of VPM thalamocortical optogenetic stimulation at the near-spindle frequencies (Bonferroni-corrected
p<0.001). (A) BOLD
activation maps for different durations of spindle-frequency stimulation an (B) BOLD activation maps for stimulation at different frequencies. 24 pulses and 8Hz stimulation is
the most efficient in evoking the brain-wide BOLD activation pattern. (C) Atlas for brain regions activated by
spindle-like stimulation. (D) BOLD profiles for 24 pulses 8Hz stimulation extracted
from atlas-based ROIs.

Electrophysiology recording locations and LFPs
evoked by different stimulation frequencies and durations (n=3, error
bar indicates ± SEM). (A) Recording
sites and averaged LFPs evoked by 24 pulses stimulation at different
frequencies. Amg, PrL, RS, and SC did not follow the 20Hz stimulation well. (B)
Averaged LFPs evoked by different durations of 8Hz stimulation. LFPs evoked by 96 pulses stimulation are weak in Amg, PrL, RS, and SC. (C) Raw LFP at S1BF with 24 pulses 8Hz stimulation. Stimulation evoked a slow oscillatory response together with the spindle-like activity.

Visual fMRI experimental design and comparisons
of activation maps and BOLD profiles (error bar indicates ± SEM, two-tail paired
sample t tests; *, ** and *** denote P < 0.05, P < 0.01 and P < 0.001). (A) Visual fMRI experimental design and
timeline. (B) BOLD activation
maps and profiles for OG group. The spatial extent of the activations and the areas under the curves of BOLD profiles showed
increased responses in SC and activated higher-order regions after stimulation. (C) BOLD activation maps and profiles
for Sham animals, no significant changes were found in SC and higher-order regions.
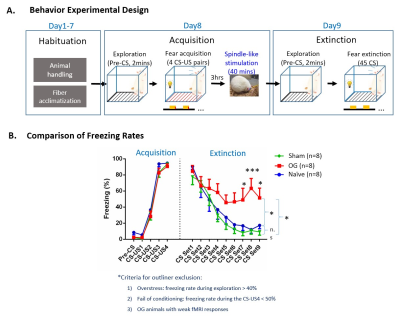
Behavior experimental design and comparisons
of freezing rate (error
bar indicates ± SEM, two-way ANOVA; * and *** denote P < 0.05 and P <
0.001). (A) Experimental design and
timeline (CS, 10s 5Hz light flash; US, unconditioned
stimulus, foot shock). (B)
Comparison of freezing rates during acquisition and extinction of
visual-somatosensory fear memory. Freezing rates of OG animals were
elevated much longer than Sham and Naïve animals, reflecting higher
resistance of its memory to extinction, indicating facilitated
memory consolidation introduced by spindle-like OG stimulations.