1352
Brain-wide functional mapping of the entorhinal cortex in young 3xTg mouse model for Alzheimer’s disease
Francesca Mandino1,2, Ling Yun Yeow2, Chai Lean Teoh2, Chun-Yao Lee2, Renzhe Bi2, Hasan Mohammad2, Sejin Lee2, Han Gyu Bae2, Seung Hyun Baek2, Hanqing Jasinda Lee3, Kim Peng Mitchell Lai3, Sangyong Jung2, Fu Yu2, Malini Olivo2, John Gigg1, and Joanes Grandjean4
1Faculty of biology, medicine and health, University of Manchester, manchester, United Kingdom, 2Singapore Bioimaging Consortium, A*STAR, Singapore, Singapore, Singapore, 3Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, 4Department of Radiology and Nuclear Medicine & Donders Institute for Brain, Cognition, and Behaviour, Donders Institute, Radboud University Medical Centre, Nijmegen, Netherlands
1Faculty of biology, medicine and health, University of Manchester, manchester, United Kingdom, 2Singapore Bioimaging Consortium, A*STAR, Singapore, Singapore, Singapore, 3Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, 4Department of Radiology and Nuclear Medicine & Donders Institute for Brain, Cognition, and Behaviour, Donders Institute, Radboud University Medical Centre, Nijmegen, Netherlands
Synopsis
Alzheimer’s disease (AD) is characterised by progressive memory loss, neurodegeneration and brain atrophy. Intra- and inter-regional connectivity across the brain is affected in AD, probably due to the aberrant accumulation of toxicity. The entorhinal cortex is a key region involved in the early stages of AD. We report synaptic connectivity increase in the 3xTg mouse model, by means of electrophysiological recordings in AD-susceptible brain regions, following stimulation of the entorhinal cortex, in vivo. Further, we demonstrate loss of functional connectivity with resting-state fMRI in AD-vulnerable brain regions, which converts into increased response during optogenetics photostimulation of the entorhinal cortex.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease, resulting in cognitive decline, memory loss, emotional and behavioural changes. The progressive neurodegeneration and brain atrophy may be caused by the synergistic interaction of neuronal hyperexcitability and the toxic accumulation of insoluble beta-amyloid plaques and neurofibrillary tangles of protein tau. This results in early damage in major hubs for cognitive function within the medial temporal lobe, particularly the lateral entorhinal cortex (ENTl), hippocampal formation and basolateral amygdala (BLA). Recently, research has shifted the attention towards investigating the early stages of pathology, where only precursor forms of plaques and tangles might appear 1. Animal models, which recapitulate aspects of AD continuum, have proven useful in exploring underlying mechanisms associated with the disease, such as depicting synaptic connectivity and whole-brain functional changes by means of electrophysiology and ultra-high field fMRI, respectively. The 3xTg mouse model for AD 2 is an ideal candidate model to investigate early brain changes at the onset of AD-like pathology, in that it is unique in developing both tangles and plaques late in life yet providing an extended time window with memory deficits occurring before tangles and plaques accumulation. 3xTgAD mice show precursor forms of tau tangles in the BLA by 3 months of age (Figure 1a), extending to the hippocampal areas by 6 months of age (Figure 1b), bringing supporting evidence for a correlation of tauopathy with memory impairments 3.Methods
Synaptic connectivity changes were assessed through electrophysiological recordings of field excitatory postsynaptic potentials (fEPSP) following ENTl stimulation, in BLA and dentate gyrus (DG). Furthermore, whole-brain resting-state functional connectivity (FC) 4 was assessed with fMRI, longitudinally at 3 and 6 months of age. Finally, we used functional mapping of the brain-wide impact of activating ENTl output by optogenetically exciting ENTl during simultaneous fMRI (ofMRI) 5,6. Paired-pulse (PPS; providing pairs of stimuli at different intervals) and input/output (input/output curve, IOC; stimulus with increasing current amplitude) stimulation protocols were provided to the ENTl, in 3xTgAD and wildtype, at 3 and 6 months of age in vivo. Secondly, nineteen 3xTgAD and ten wildtype mice underwent longitudinal resting-state fMRI at 11.75 T using a cryogenic receiver surface coil (2x2 phased-array receiver only coil), at 3 and 6 months of age. Lastly, twelve 3xTgAD and ten controls were stereotactically injected with Channelrhodopsin-2 (ChR2, AAV5-CamKIIa-hChR2-mCherry) in the ENTl, whereas nine mice were injected with mCherry-alone as a negative control. An optical implant was inserted and secured with dental cement. Injected mice underwent fMRI scan using a 10 mm surface receiver coil. Gradient-echo echo-planar images for resting-state fMRI were acquired with the following parameters: TR = 1000 ms, TE = 15 ms, FA = 50°, matrix 90 x 60, FOV 17 x 9 mm2, slice thickness 0.35 mm. The parameters varied for ofMRI experiments: TR = 1000 ms, TE = 11.7 ms, FA = 50°, matrix 60 x 30, FOV 17 x 9 mm2, slice thickness 0.45 mm. ChR2 was photostimulated with 10 s blocks of 473 nm blue light, at 5, 10 and 20 Hz in random order, with 10 ms pulse width. All animals were imaged under isoflurane 0.5%, medetomidine 0.1 mg/kg/h, and muscle relaxation 7. In the resting-state experiments, regional homogeneity (ReHo 8) analyses were used to investigate local functional connectivity and network analysis was performed on the whole-brain level and in relation to the ENTl. In stimulus-evoked condition, a general linear model (GLM) analysis with gamma function was applied.Results
Electrophysiological recordings in the BLA and DG show augmented synaptic excitability (DG is reported as an example in Figure 2a). Slice electrophysiological recordings in DG confirmed augmented spiking activity in 3xTgAD (Figure 2b). Brain-wide assessment of FC revealed network and localised loss in regions highly involved in episodic memory, emotional processing and reward, i.e. BLA, nucleus accumbens within the ventral striatum, ENTl and hippocampal areas (Figure 3). The optogenetic activation of ChR2 in entorhinal pyramidal neurons showed a faithful optogenetically-locked BOLD response in both 3xTgAD and wildtype cohorts (Figure 4d,e,f), whereas mCherry-alone mice did not show light-induced BOLD response (Figure 4f). Interestingly, 3xTgAD responses were significantly higher than controls, at both ages (Figure 5). We conclude that, by 3 months of age, 3xTgAD mice show decreased functional coupling in the entorhinal cortex during spontaneous activity. This converts to increased activity in major hubs for tauopathy upon strong experimental activation, thus suggesting an increase in metabolic demand, possibly resulting from heightened synaptic activity.Discussion & Conclusion
This dichotomy between rest and induced activity might be resolved by considering a state where there is an overall decrease in inter-regional axonal connectivity that occurs alongside an increase in synaptic strength for the remaining connections in the 3xTgAD model. During basal activity, deficits in connectivity could explain the decreased resting-state activity, whilst the increased synaptic activity and short-term plasticity produced by induced, synchronous activation could potentially explain the apparently incongruous hyperexcitability seen during electrical and optogenetic activation of the entorhinal cortex. Thus, here we demonstrate a brain-wide reorganisation in young 3xTgAD mice, prior to plaques and tangle deposition, in line with clinical evidence and in further support of the co-occurrence of network and neuronal dysfunction with early stages of tauopathy.Acknowledgements
This work was supported by the A*STAR Research Attachment Programme (ARAP), which is co-funded through the University of Manchester, Faculty of Biology, Medicine and Health Doctoral Academy, and Singapore Bioimaging Consortium (SBIC), A*STAR, Singapore.References
- Jack Jr CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. The Lancet Neurology. 2010;1;9(1):119-28.
- Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;31;39(3):409-21.
- Davis KE, Easton A, Eacott MJ, et al. Episodic-like memory for what-where-which occasion is selectively impaired in the 3xTgAD mouse model of Alzheimer's disease. Journal of Alzheimer's Disease. 2013;1;33(3):681-98.
- Biswal B, Zerrin Yetkin F, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic resonance in medicine. 1995;34(4):537-41.
- Grandjean J, Corcoba A, Kahn MC, et al. A brain-wide functional map of the serotonergic responses to acute stress and fluoxetine. Nature communications. 2019;21;10(1):350.
- Kahn I, Knoblich U, Desai M, et al. Optogenetic drive of neocortical pyramidal neurons generates fMRI signals that are correlated with spiking activity. Brain research. 2013;20;1511:33-45.
- Grandjean J, Schroeter A, Batata I, et al.Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage. 2014;15;102:838-47.
- Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;1;22(1):394-400.
Figures
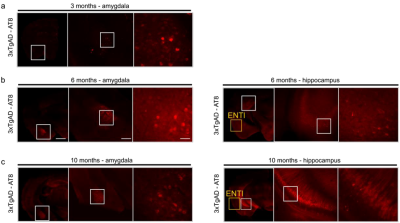
Figure 1: Immunohistochemistry for 3xTgAD
characterisation with AT8. a) 3xTgAD
mice show phosphorylated tau in the amygdala by 3 months. b) 6 months old 3xTgAD mice show tau-positive labelling in the
amygdala and extended to the hippocampus; ENTl shows no apparent pathology. c) 10 months old 3xTgAD mice show phospho-tau
staining in the amygdala and hippocampus. ENTl however does not show positive
response to AT8. left panels 1000 μm, middle panels 500 μm, right panels 100
μm.
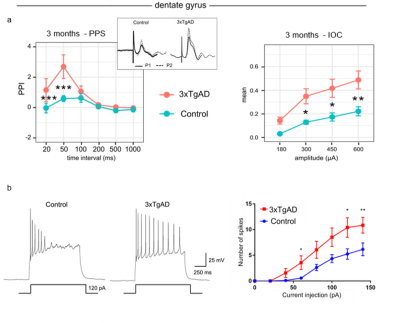
Figure 2: Electrophysiological evidence in vivo and vitro. a) Left: fEPSP
response to PPS recorded in DG curve in 3xTgAD 3 months old mice and controls
showed a significant increase in facilitation at short intervals: 20 ms, 50 ms.
Right: IOC for 3-month-old 3xTgAD mice and matched controls in DG shows
significant difference is excitability in 3xTgAD compared to controls. b) Firing
pattern of action potential with 120 pA injection (left) and number of spikes
(right) in granule cells of DG.
Data are plotted as mean ± SEM.

Figure 3: Resting-state fMRI shows regional homogeneity
decrease in 3xTgAD. a) Regional Homogeneity (ReHo) shown as
thresholded group analysis for 3 months and 6 months of age at left and right,
respectively. 3xTgAD mice show a significant decrease in ReHo by 3 months of
age within the ENTl, ACB and BLA. 3xTgAD shows increased ReHo values for
SSp-bfd, by 6 months of age. b,c) A
significant decrease in ReHo was found at 3 and 6 months of age in the ENTl. d) Pair-wise ROI interactions relative
to the left ENTl shows decrease of FC in right ENTl, ACB, BLA.
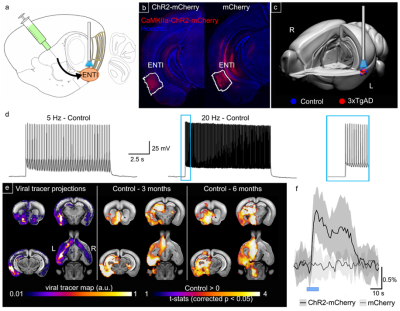
Figure 4: Optogenetic
modulation of the ENTl. a) Stereotactic surgery. b) ChR2-mCherry/Hoechst in ChR2-mCherry and mCherry. c) 3D rendering of fibre position. d) Frequency-locked spiking activity in vitro at 5 and 20 Hz in
control mice. e) Optogenetically-locked
BOLD response overlaps with density projection maps, highlighting activation in
key regions related to ENTl projections, i.e. hippocampal formation, BLA, nucleus
accumbens. f) Average of BOLD response to 20 Hz stimulation, in ChR2-mCherry and mCherry
alone mice
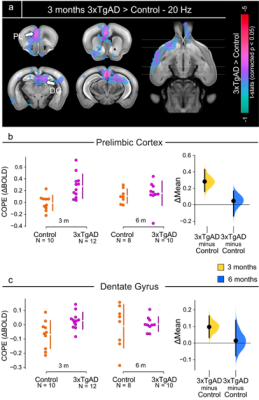
Figure 5: Two-sample t-test results, 3 months
time-point.
a) Two-sample
t-test showing significantly higher response (p < 0.05, corrected) in 3xTgAD
compared to controls in regions encompassing the prefrontal cortex, such as prelimbic
(PL), dentate gyrus (DG) and ventral striatum (nucleus accumbens, ACB). b,c) COPE extracted from the PL and DG
as an example of potentiated response in 3xTgAD compared to age-matched controls,
at 3 and 6 months of age. COPEs: contrast of parameter estimates.