1351
Functional dissection of somatosensory processing pathways in mice1Cener for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon-si, Gyeonggi-do, Korea, Republic of, 2Department of Biomedical Engineering, Sungkyunkwan University, Suwon-si, Gyeonggi-do, Korea, Republic of
Synopsis
Somatosensory system is communicated by feedforward and feedback projection each other during functional processing. Somatosensory fMRI response is attributed to these inter-regional reciprocal projections. Therefore, the separation of functional pathways in fMRI data is important to interpret fMRI data in circuit level. Here, to dissect somatosensory fMRI response, we compared CBV-weighted fMRI obtained at 15.2T under three conditions: excitation by sensory stimulation, silencing of somatosensory cortex by optogenetic stimulation, and combined excitation and silencing.
Introduction
Recently, ultrahigh-field fMRI in lightly anesthetized mice showed functional activities in somatosensory networks, consistent with the anatomical neural tracing map1. In the somatosensory network, the thalamic nuclei not only transmit external signals to the cortex, but also receive the cortical feedback projection. In addition, the cortical areas are reciprocally projected each other2. These inter-regional connectivities during stimulation may complexly contribute to fMRI. To determine fMRI maps in a circuit level, it is important to separate the contribution of thalamo-cortical (TC), cortico-thalamic (CT) and cortico-cortical (CC) pathways. For this, optogenetics is an excellent tool for selectively modulating specific circuits3. Here, we dissected fMRI responses without and with silencing neural activities of the primary somatosensory cortex by optogenetic stimulation of interneurons.Materials & Methods
Eight transgenic mice (6-7 weeks, 21-27g) expressing light-sensitive opsin proteins in the GABAergic interneuron populations (Vgat-ChR2-EYFP) were used to selectively drive inhibition on the local pyramidal neuronal activity by optical stimulation4. Optical fiber cannula (Ø105 µm core) was implanted into the primary somatosensory cortex (AP: -0.2 mm, ML: +2.2 mm and DV: +0.5 mm). After a recovery period of at least 2 weeks, fMRI experiments were performed under ketamine-xylazine anesthesia5.Since EPI at an ultrahigh field is susceptible to large distortions and signal loss (Fig.1Ai), the conventional gradient echo-based imaging technique with short echo time (Fig.1Aii) was used for fMRI after the injection of monocrystalline iron oxide nanoparticles (MION, 45 mg/kg) (Fig.1Aiii). CBV-weighted fMRI data were acquired on 15.2T/11cm Bruker BioSpec using the FLASH sequence with TR/TE=50/3ms, flip angle=15°, spatial resolution=156×156x500μm3, 6 coronal slices and temporal resolution=2s. For fMRI studies, we designed three stimulus conditions (Fig.1B): excitation by forepaw stimulation (FP; 4 Hz, 0.5 ms, 0.5 mA), silencing of S1 activity by optogenetic stimulation (OG; 473 nm blue light, 20 Hz, 10 ms, 3 mW at tip, time-averaged power ≈ 69.3 mW/mm2), and combined excitation and silencing (FP+OG). Each stimulus run consisted of 110 volumes: 30 pre-stimulus-10 stimulus-30 rest-10 stimulus-30 post-stimulus. For each stimulus condition, 15 fMRI trials were obtained for signal averaging.
First, fMRI activation map by forepaw stimulation was generated using GLM analysis, and 6 different ROIs were defined based on the Allen mouse brain atlas6; S1FL, M1, S2, medial-, posterior- and ventral-thalamus (Fig.2Ai). Second, CBV-weighted signal changes within each ROI under three conditions were compared to determine the contribution of TC and CT/CC pathways (Fig.4). Third, M1 and S2 were flatted7 and cortical-depth profiles were plotted to measure the layer-dependent distribution of input from TC & CC projections to M1 and S2 activity (Fig.5).
Results
Animal-wise averaged fMRI maps by forepaw stimulation and time courses (Fig.2) showed CBV increases in cortical areas (S1FL, M1 and S2) and thalamic nuclei (mTh, PO and VPL). In contrast, optogenetic S1 silencing produced the CBV decrease in the same regions (Fig.3A-i and B), indicating that the S1 projections to those areas were reduced relative to basal condition. Interestingly, when it combined with forepaw stimulation, the CBV response in VPL returned to baseline, whereas the other areas still remained the decreased CBV (Fig.3Aii and B).Functional changes responding to three different paradigms were quantified (Fig.4), and feedforward contribution was determined by the difference between the FP+OG and OG stimulation. Then, other contributions were calculated from ratio of sensory-evoked signal by FP stimulation to feedforward contribution. The somatosensory-induced fMRI in VPL is mostly from the TC contribution (~85%), while the responses in other ROIs are mostly (>70%) from the S1 projection (Fig.4vi).
The separation of TC and CC contributions to cortical activities was determined by layer-dependent analysis (Fig.5A). The highest CBV response to forepaw stimulation at S1FL was detected in layer IV, indicating dominant TC contribution, whereas M1 and S2 responses were peaked at layer II/III (Fig.5Bi), suggesting CC projections. Similar layer-specific responses in M1 and S2 were observed during optogenetic S1 silencing (Fig.5Bii).
Discussion & Conclusion
In this study, we demonstrated the functional dissection of mouse somatosensory networks using high-resolution CBV fMRI at ultrahigh-field of 15.2T combined with optogenetics. The major findings were:1) Thalamic nuclei are functionally connected with S1FL for somatosensory processing, as measured by fMRI. VPL plays a role in relaying sensory input to S1FL, while the PO activity is driven by the S1FL projection. It is consistent with neurophysiological findings, which show the suppression of evoked neural activity in PO, but not ventral thalamic nuclei, in response to simultaneous whisker stimulation and cortical inactivation8.
2) The sensory-evoked responses in S1, S2, M1 and mTh are mainly contributed by S1FL projections. In our layer analysis, M1 and S2 activities are likely due to the direct CC projection. The M1 and S1 layer finding is supported by the laminar profiles in S1FL and M1 measured by simultaneous electrophysiological recordings during forepaw stimulation9. However, since those areas also receive afferents from S1FL indirectly (e.g., S1F->PO->S2, S1FL->M1->mth)3, further systematic studies are necessary for understanding the functional pathways of somatosensory networks.
The high-resolution CBV fMRI was successfully detected with the minimal BOLD contribution due to the use of short TE and large MION dose. Ultra-high-field fMRI of transgenic mice in combination of cell-type specific optogenetic and chemogenetic stimulation will provide circuit-level pathways in whole brain.
Acknowledgements
This work was supported by IBS-R015-D1.References
1. Jung WB, Shim HJ, Kim SG. Mouse BOLD fMRI at ultrahigh field detects somatosensory networks including thalamic nuclei. Neuroimage. 2019;195:203-214.
2. Feldmeyer D, Brecht M, Helmchen F, et al. Barrel cortex function. Prog Neurobiol. 2013;103:3-27.
3. Lee JH, Durand R, Gradinaru V, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465(7299):788-92.
4. Zhao S, Ting JT, Atallah HE, et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8(9):745-52.
5. Shim HJ, Jung WB, Schlegel F, et al. Mouse fMRI under ketamine and xylazine anesthesia: Robust contralateral somatosensory cortex activation in response to forepaw stimulation. Neuroimage. 2018;177:30-44.
6. Oh SW, Harris JA, Lydia NG, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508(7495):207-14.
7. Shih YY, Chen YY, Lai HY, et al. Ultra high-resolution fMRI and electrophysiology of the rat primary somatosensory cortex. Neuroimage. 2013;73:113-20.
8. Mease RA, Sumser A, Sakmann B, et al. Cortical dependence of whisker responses in posterior medial thalamus in vivo. Cereb Cortex. 2016;26(8):3534-43.
9. An S, Kilb W, Luhmann HJ, et al. Sensory-evoked and spontaneous gamma and spindle bursts in neonatal rat motor cortex. J Neurosci. 2014;34(33):10870-83.
Figures
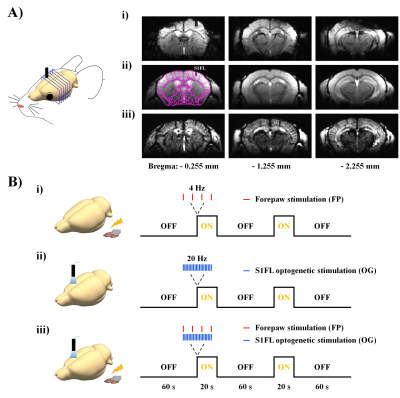
Figure 1. MR image quality and experimental designs.
A) High-resolution mouse brain images using i) single-shot gradient-echo EPI with TR/TE=1000/12ms and FLASH with TR/TE=50ms/3ms ii) without and iii) with MION.
B) Experimental designs for excitation and silencing of somatosensory networks: i) excitation by 4-Hz forepaw stimulation, ii) silencing of S1 activity by 20-Hz optogenetic stimulation and iii) combined excitation and silencing.
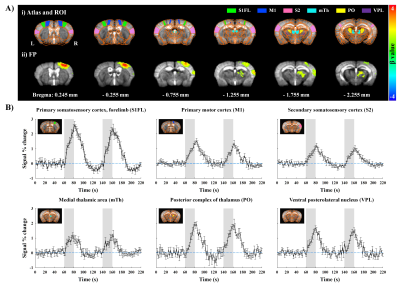
Figure 2. Functional maps and time courses measured by CBV-weighted fMRI responding to 20-s forepaw stimulation in lightly anesthetized mice.
A-i) Atlas-based ROI definitions and A-ii) animal-wise averaged fMRI maps (FWE corrected p < 0.05).
B) Time courses in cortical and thalamic areas (mean±SEM). Area in shade indicates the 20s duration of stimulation. Note that original negative changes were plotted as positive CBV changes.
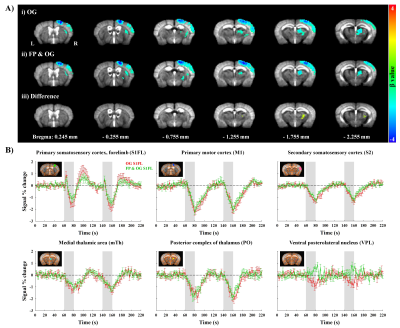
Figure 3. fMRI of optogenetic S1FL silencing without and with forepaw stimulation.
A) Group-averaged fMRI maps during i) optogenetic S1FL silencing and ii) combined FP and OG stimulation (FWE corrected p < 0.05) and the difference map between two stimulus conditions (uncorrected p < 0.01)
B) Time courses responding to optogenetic S1FL silencing without and with forepaw stimulation in cortical and thalamic areas (mean±SEM). Area in shade indicates the 20 s duration of stimulation.
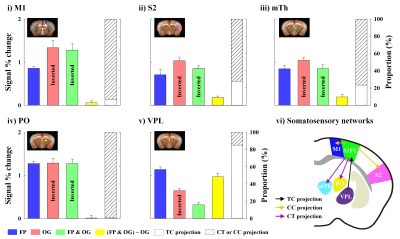
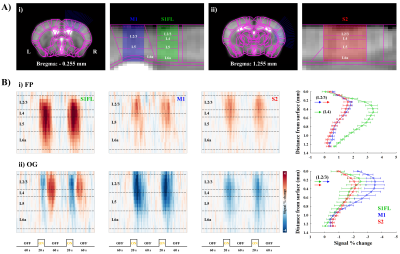
Figure 5. Laminar analysis of CBV response during forepaw and optogenetic stimulation
A) Linearization of cortical areas of i) S1FL, M1 and ii) S2. The definition of cortical layers was based on the Allen mouse brain atlas.
B) Cortical depth-dependent CBV time courses (left) and laminar profiles (right) of S1, M1 and S2 during i) forepaw and ii) optogenetic S1 stimulation.