1348
Advanced methods for concurrent TMS/fMRI explain target engagement in 10Hz rTMS treatment1Medical University of Vienna, Vienna, Austria
Synopsis
We have established and validated a concurrent TMS/fMRI setup to study target engagement of TMS-treatment during stimulation. The proposed marker for target engagement is a change in anti-correlation of the sgACC to the DLPFC. The direct sgACC effect due to DLPFC stimulation can only be observed by concurrent fMRI. We could show that TMS treatment over the left DLPFC leads to lasting effects in RS connectivity and importantly overlap with acute BOLD response during stimulation. We conclude that concurrent TMS/fMRI can be used to investigate efficacy of treatment and thereby propose a translation into clinical medicine.
Introduction
Transcranial magnetic stimulation in clinical medicine is one of the top most promising tools for depression treatment. Although the method has been FDA-approved, important information on target-engagement is missing. In depression it has been suggested that the best predictor for treatment efficacy is reflected in the anti-correlation between the subgenual ACC and the DLPFC before stimulation and a decrease of anti-correlation afterwards as indicator for treatment response1,2. This mechanism of action has, however, never been validated in healthy subjects and the expected target engagement has not been shown to be a direct effect of stimulation. We have recently shown a connectivity increase after 10Hz TMS over left DLPFC in a healthy sample3. To bridge the gaps concerning insights on target engagement, we have: reanalyzed the data of our first study in terms of anti-correlations and designed a second study utilizing an in-scanner TMS/fMRI setup that would allow for precise investigation on target engagement during stimulation based on these former results. Thereby we have advanced the method for increased stimulation accuracy & monitoring. In the course of this project we have reconsidered different approaches for sham control for combined TMS/fMRI and developed a method to use neuronavigation even inside the scanner bore.Methods
Reanalysis RS (Study 1). To analyse lasting TMS effects, a sample of 60 healthy right-handed subjects (31 female, age: 25.01 ± 4.6 years) was stimulated over left DLPFC at 90% of rMT, frequency of 10Hz, 1200 pulses. For sham stimulation the vertex was stimulated. Resting-state fMRI was performed pre and post stimulation. Functional imaging was acquired employing a single-shot gradient-recalled EPI sequence (TR/TE = 1800/38 ms, matrix = 128 × 128, 23 axial slices parallel to the AC-PC-plane, voxel size 1.5 x 1.5 x 3 mm3, slice gap: 1.8 mm). During RS fMRI, participants were instructed to look at a fixation cross and let their mind wander. We then investigated network effects after TMS in 20 most common resting state networks4.Target Engagement TMS/fMRI (Study 2). To investigate acute TMS/fMRI-effects, 14 right-handed subjects (6 male, age: 28 ± 3.9 years) participated in a second experiment. The study was performed on a 3T Prisma scanner (Siemens, Erlangen, Germany) using a TMS/fMRI setup comprising the MagProX100 stimulator with an MRi-B91 MR-compatible TMS coil (Magventure, Farum, Denmark). Functional images were acquired using the CMRR EPI sequence with TR/TE=1000/38ms, 36 slices, 3 x 3 x 3mm³, 20% slice gap, MB-factor=4, delayinTR=320ms. The TMS/fMRI coil-array was positioned over the left DLPFC using an MR-compatible neuronavigation system. The stimulation target was at Brodmann area 46 (MNI-coordinate: -42, 28, 21 5).
TMS/fMRI protocol. Concurrent TMS/fMRI was applied at 10Hz triplets at four different intensities relative to the individual motor threshold. We used a 320 ms long acquisition gap in order to prevent artifacts in EPI6.
Neuronavigation. In order to accurately position the TMS coil before entering the scanner bore, we have developed an MR-compatible neuronavigation system. This required us to reproduce subject and coil trackers from non-ferrous material using precise 3D-printing. Beyond that we have designed a subject tracker holder, which is tailored to the individual subjects anatomy and placed in their mouth to keep it stable and optimally tilted (Figure 3).
Data analysis. FMRI data analyses were performed using SPM12. The design matrix comprised four regressors representing different stimulation amplitudes (80%, 90%, 100%, 110% of the individual’s rMT) of 10Hz TMS triplets over the left DLPFC. The 4 beta maps per subject went into a second-level factorial design.
Sham conditions. For the reanalysis study we had opted to perform sham stimulation over vertex. However, this procedure was recently shown to influence the default-mode network7. Based on this finding we wanted to design a sham condition for concurrent TMS/fMRI, where the stimulation coil has the same position as in real stimulation and that provokes similar acoustic and sensory effects. Therefore we placed an empty TMS stimulator housing between TMS-coil and RF-coil and validated this procedure as a method for placebo stimulation (Figure 3).
Results
Reanalysis RS (Study 1). We were able to show a decrease in anti-correlation of the sgACC after 10 Hz stimulation to RSN#17.Target Engagement TMS/fMRI (Study 2). During left DLPFC stimulation an increase in sgACC (T=3.6, p<.05 cluster-level FWE-corrected) activity could be observed for 110%>80% rMT stimulation. Furthermore significant activations in the network hubs of the RSN#17 were detected over stimulation intensities.
Sham stimulation (pilot data) resulted in the activation of a somato-sensory and auditory network and did not lead to significant stimulation effects in targeted left DLPFC.
Discussion
Here, we have shown that TMS increases sgACC correlation after stimulation (RS post>pre rTMS).This can be attributed to direct BOLD changes during stimulation as measured using an advanced TMS/fMRI setup with neuronavigation at the scanner bore.
In the course of this study we were able to develop a placebo condition using a sham-coil array, which as pilot data suggests does activate sensory areas without actively influencing the target of interest.
Conclusion
Standardized in-scanner placebo-controlled target engagement examinations before TMS depression treatment can provide clinical practitioners with knowledge on optimal patient specific parameters (e.g. dose) and expected treatment response, once this method is translated to clinical practice.Acknowledgements
No acknowledgement found.References
- Fox, M. D., Liu, H. & Pascual-Leone, A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage 66, 151-160 (2013).
- Williams, N. R. et al. High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain (2018).
- Tik, M. et al. Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes network-specific increase in functional connectivity. NeuroImage 162, 289-296 (2017).
- Biswal, B. B. et al. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences 107, 4734-4739 (2010).
- Fox, M. D., Halko, M. A., Eldaief, M. C. & Pascual-Leone, A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62, 2232-2243 (2012).
- Navarro de Lara, L. I. et al. A novel coil array for combined TMS/fMRI experiments at 3 T. Magnetic resonance in medicine 74, 1492-1501 (2015).
- Jung, J., Bungert, A., Bowtell, R. & Jackson, S. R. Vertex stimulation as a control site for transcranial magnetic stimulation: A concurrent TMS/fMRI study. Brain stimulation 9, 58-64 (2016).
Figures

Figure 1. rTMS- and acute TMS/fMRI effects on sgACC. Activation changes during TMS (right column) explain lasting changes on network connectivity (left column).
(Study 1, left) Compared to pre rTMS (RS1, middle) the anticorrelated sgACC shows increased correlation post rTMS (RS2) in line with Fox et al. (2012, 2013).
(Study 2, right) F-contrast over all stimulation intensities during concurrent TMS/fMRI (p<0.05,FWE). As shown in the bottom row, TMS/fMRI led to increased activity in the sgACC for 110%>80% stimulation intensity explaining longer lasting changes on RS connectivity.
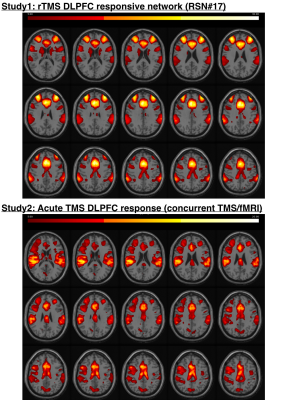
This figure shows the overlap between acute TMS effects (14 subjects) and TMS responsive network (60 subjects). Stimulation led to increased activation in bilateral DLPFC, IPL, ACC and caudate nuclei (Study1, bottom) as measured by concurrent TMS/fMRI. These are core components of the TMS responsive RSN#17 (Study2, top).
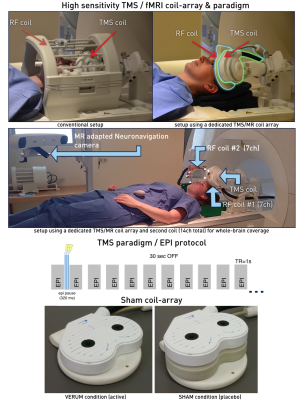
Figure 3. Concurrent TMS/fMRI setup. Upper panel shows comparison between the conventional low sensitivity TMS/fMRI setup within a bird-cage coil (left) and our developed ultra-sensitive concurrent TMS/fMRI coil array (right).
The middle panel shows the advanced navigated TMS/fMRI setup. Importantly a second RF coil now allows for whole-brain coverage. As depicted below, the concurrent EPI acquisition timing allowed to trigger 3 TMS pulses during acquisition gap between volumes.
The bottom panel shows our new TMS/fMRI coil array for verum (left) and sham condition (right).
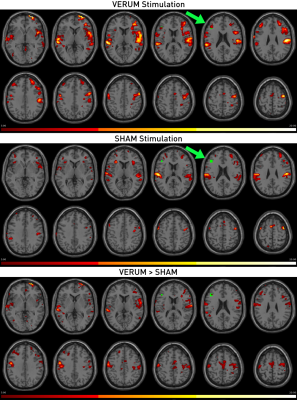
Figure 4. Single-subject activation changes for VERUM (active) vs. SHAM (placebo) stimulation.
This figure shows the results for an individual subject stimulated at a dose of 100% with the standard verum TMS/fMRI setup and the sham setup (see Figure 1). While both conditions evoked somatosensory activations in the supramarginal gyri and anterior insulae, only real stimulation led to activation changes in the target (green), i.e. left DLPFC (p<0.05, FWEwholebrain corrected).