1344
Evaluation of an improved microelectrode array for MR-compatibility and MR-simultaneous recording performance in 7T research system1Interdisciplinary Institute of Neuroscience and Technology, School of Medicine, Zhejiang University, Hangzhou, China, 2Department of Neurology of the Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou, China, 3Department of Biomedical Engineering, National Yang Ming University, Taipei, Taiwan, 4College of Biomedical Engineering and Instrument Science, Zhejiang University, Hangzhou, China
Synopsis
Simultaneous recording of electrophysiological signals with functional magnetic resonance imaging (fMRI) can provide a solution for investigation of neurovascular coupling. However, this technique is challenged by 2 aspects, image artifact from electrode and electrophysiological noise from magnetic field. We improved our pervious lab-designed microelectrode array and developed a de-noise method for use of its electrophysiological recording in 7T MRI. The results showed better structural image quality and stable acquisition of spike signals and local field potential. The proposed tool and method has the potential to facilitate simultaneous spike-recording during MR scanning in 7T MRI and further study the neurovascular coupling.
Introduction
Many questions are unsolved concerning the relationship between cerebral hemodynamic change and neural activity. For investigation of brain function, a thorough understanding of neurovascular coupling has the potential to link human studies based on functional magnetic resonance imaging (fMRI) with a large body of animal research based on neural electrophysiological recording, bridging the gap between deduced brain activation and neural firing events in human studies due to avoiding invasive method.1-5 Simultaneous recording of electrophysiological signals with fMRI can facilitate the research of neurovascular coupling. However, this technique is limited by aspects such as image artifact brought by the microelectrode array and electrophysiological noise induced by gradient and radio-frequency pulses during MR scanning.1,6,7 The present study proposed an improved lab-designed MR-compatible microelectrode array and evaluated its MR-compatibility and performance of electrophysiological recording during 7T MRI scanning.Methods
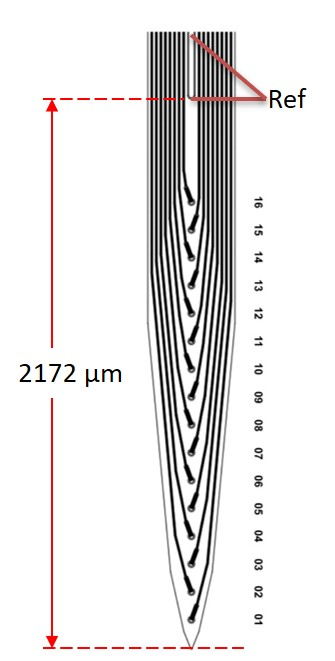
To reduce image artifact of microelectrode array and reference screw, the manufacturing process was modified and a reference electrode was added above the 16 recording microelectrodes on the microelectrode array. The reference electrode replace the function of reference screw (Figure 1). The cats were anesthetized with 2% isoflurane and a craniotomy was made at either of two target sites: (1) 5-mm anterior to bregma and 5-mm lateral to midline, (2) 2-mm anterior to ear bar and 4-mm lateral to midline for implantation of microelectrode arrays. During MR scanning and electrophysiological recording, the animal was anesthetized with 0.3 % isoflurane and intravenously (i.v.) injected ketamine (8 mg/kg*h). To exam the image artifact of microelectrode array, whole-brain T1-weighted images (T1-WIs) by a turbo spin echo (TSE) sequence (TR=3300 ms, TE=18 ms, BW=100 Hz, voxel size: 0.5×0.5×0.5 mm3) were obtained using 7T research system (Siemens, Erlangen, Germany). The neural electrophysiological signals were recorded by MR-compatible neurophysiological system (TDT, Alachua, USA) (filter: 300 - 5k Hz, sampling rate: 25 kHz) before, during and after scanning of an echo-planar imaging (EPI) sequence (TR=2000 ms, TE=24.2 ms, voxel size: 1.5×1.5×1.5 mm3, 144 measurements). The trace of the microelectrode array in T1-WIs was compared with that of our previous microelectrode array. Using the ensemble empirical mode decomposition (EEMD) removed the electrophysiological noise induced by alterations of MR gradient field, and the then the spikes were sorted and temporal-frequency spectra were analyzed.Results and Discussion
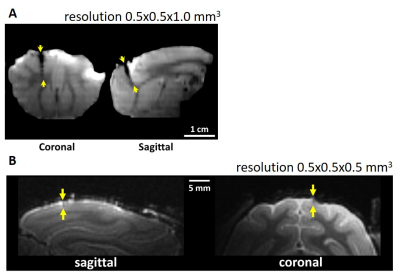
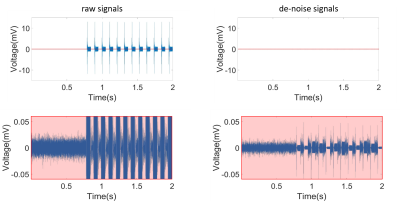
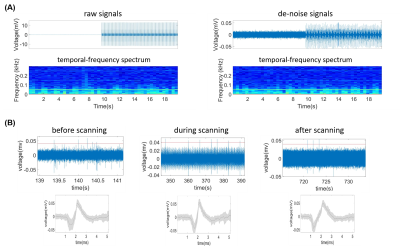
Figure 2 showed the coronal and sagittal planes reconstructed from the T1-WI of previous microelectrode array (Figure 2A) and modified microelectrode array (Figure 2B). The results showed image artifact of the improved microelectrode array obviously diminished as compared with previous microelectrode array, implying that the influence of our microelectrode array on structural imaging under 7T is acceptable. Figure 3 showed the de-noise method using EEMD could remove the MRI interference from electrophysiological signals during 7T MR scanning. Figure 4 showed the electrophysiological signals and temporal-frequency spectrum before and after de-noise process. The gradient noise is removed while the frequency band of local field potential (LFP) is retained. This will benefit the extraction of LFP signal during EPI scanning in the future. After de-noise of gradient field artifact, the spikes detected and sorted before, during and after EPI scanning were plotted, as showed in Figure 4B. The amplitudes and shapes of the spike waveforms before and after scanning are similar, implying that they came from the same firing unit. This suggests that this microelectrode array can acquire spike signals throughout the functional imaging process with decent quality in 7T scanner.Conclusion
This study proves that our improved microelectrode array is MR-compatible to 7T MRI, providing better structural image quality and stable acquisition of spike signals, and the EEMD de-noise method could be effective removal of signal artifact by MRI. These advantages endow it with potential to investigate the relation between single-unit spike signal and BOLD signal simultaneously, furthering the understanding of coupling between neural and hemodynamic activities.1-5Acknowledgements
This work was supported by grants from the Fundamental Research Funds for the Central Universities (2016QN81017) and the NationalNatural Science Foundation of China (81527901, 61673346, 81527901) . MR Collaboration, Siemens Healthcare Ltd., Shanghai, China.References
1. Logothetis N K, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature, 2001, 412(6843): 150
2. Kayser C, Kim M, Ugurbil K, et al. A comparison of hemodynamic and neural responses in cat visual cortex using complex stimuli. Cerebral Cortex, 2004, 14(8): 881-891.
3. Goense J B M, Logothetis N K. Neurophysiology of the BOLD fMRI signal in awake monkeys. Current Biology, 2008, 18(9): 631-640.
4. Zaldivar D, Logothetis N K, Rauch A, et al. Pharmaco-based fMRI and neurophysiology in non-human primates. In Vivo Neuropharmacology and Neurophysiology. Humana Press, New York, NY, 2017: 37-66.
5. Hermes D, Nguyen M, Winawer J. Neuronal synchrony and the relation between the bloodoxygen-level dependent response and the local field potential. PLoS biology, 2017, 15(7): e2001461.
6. Chen Y-Y, Lai H-Y, Lin S-H, et al. Design and fabrication of a polyimide-based microelectrode array: application in neural recording and repeatable electrolytic lesion in rat brain. Journal of neuroscience methods, 2009, 182(1): 6-16.
7. Karmarkar P V. Implantable MRI compatible stimulation leads and antennas and related systems and methods: U.S. Patent 8,509,876.2013-8-13.
Figures