1341
Adaptive Virtual Referencing Enables Recording of Extracellular Action Potentials in a 16.4 Tesla Animal Research MRI Scanner1Mechanical Engineering, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Recording neural signals such as extracellular action potentials during functional magnetic resonance imaging (fMRI) will improve our understanding of neurovascular coupling, which is responsible for the fMRI blood oxygen level dependent (BOLD) signal. Recording electrical neural signals during fMRI is challenging due to interactions between the recording hardware and electromagnetic (EM) fields involved in MRI that introduce noise and artifacts. We developed an adaptive virtual referencing technique to improve the action potential signal quality recorded in the bore of a 16.4T animal scanner during GRASE fMRI. This technique will enable us to further study neurovascular coupling at 16.4T.
Introduction
Combining extracellular recording with functional magnetic resonance imaging (fMRI) provides high spatiotemporal resolution nearby the implanted electrode recording sites as well as information about whole brain function and connectivity. The extracellular recording provides a direct measure of neuronal signaling and can be used to study the neurovascular coupling underlying the blood oxygen level dependent (BOLD) contrast mechanism 1,2. However, combining extracellular recording and MRI faces challenges including interactions of the recording hardware with the electromagnetic fields of MRI, which can induce electrical potentials that create artifacts in the extracellular recordings. A variety of techniques have been used to reduce interference observed while recording neural signals inside MRI scanners 3–7. In this abstract, we present the use of adaptive virtual referencing for multi-channel extracellular recording to reduce noise observed inside the bore of a 16.4T animal MRI scanner. This technique allows us to uncover extracellularly recorded action potentials in the bore of the scanner and during fMRI acquisition.Methods
An MRI-compatible 16-channel microelectrode array with 150 mm channel spacing (NeuroNexus) was chronically implanted into the cortex of a rat using aseptic procedures. After post-surgical recovery, the rat was anesthetized with isoflurane, head-fixed, and placed in the bore of a 16.4T horizontal-bore research scanner. A single-loop proton RF surface coil was positioned over the head. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota. A non-ferrous recording system (Blackrock Microsystems) was used to obtain extracellular recordings at 30 kHz with voltage resolution of 2 mV/bit to avoid signal saturation during scanning. Recordings were made outside and inside the magnet bore under several experimental conditions. The data were post-processed to compare signal quality, specifically focusing on the extracellular recording of action potentials.An adaptive virtual referencing approach was developed to improve the quality of the action potential signals recorded inside the magnet bore. The range over which action potentials can be recorded is estimated to be less than 140 microns 8. Therefore, the spatial average of the 16-channel recording data (each channel separated by 150 mm) preserves the common mode noise but reduces recorded action potential amplitudes below the noise floor. The spatial average was used in a two-stage multiple-input multiple-output (MIMO) adaptive filter to generate an adaptive virtual reference for each channel. The equations for the standard adaptive coefficient filter with least-mean-square coefficient update can be found in the literature 9. We used a 16-channel, two-stage filter with the first stage focusing on removing interference from the gradient amplifiers, and the second stage removing interferences from helium pump vibration. Each stage contained 6 coefficients and a step-size of 1e-6 was used.
Results
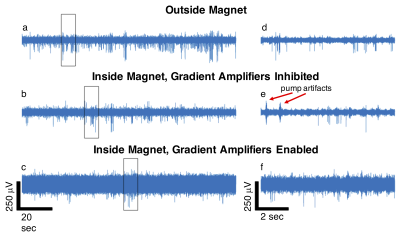
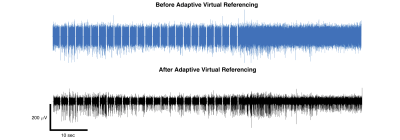
A comparison of the action potential data quality for a single channel in different environments is provided in Fig. 1. The recording outside of the magnet bore shows clear neuronal action potentials. When the rat was placed inside the magnet bore but scanner hardware was disabled, periodic helium pump vibration artifacts that were inconsistent in time and space were observed. Finally, when the magnet hardware was enabled, specifically the gradient amplifiers, a large increase in noise interference was observed that severely degraded signal quality.Our adaptive virtual referencing technique was able to substantially reduce the environmental noise and improve the ability to detect action potentials in the MRI scanner bore. An example of action potentials detected during a fMRI scan using the GRASE imaging sequence 10 is presented in Fig. 2. The signal-to-noise ratio (SNR) of the action potential recording is dramatically improved by our adaptive virtual referencing technique. A comparison of the action potential waveforms before and after adaptive virtual referencing is provided in Fig. 3.
Discussion
The increased noise level observed when recording action potentials inside the bore of the 16.4T MRI scanner is attributed to periodic artifacts from helium pump vibration and increased noise interference from enabling the gradient amplifiers. Both sources of noise were observed to be common mode and could be removed or at least substantially reduced using the newly developed 16-channel MIMO two-stage adaptive virtual referencing scheme. We were even able to reduce the neural spike detection threshold from above 500 mV to approximately 100 mV in one case, which was adequate to detect neural spikes of large amplitude (albeit with low SNR). Additionally, because we can remove the interference from the helium pump vibration, we do not have to turn off the helium pump during recording experiments. Finally, we showed that we could observe action potentials during fMRI scanning, which demonstrates promise for simultaneous fMRI and extracellular recording at ultrahigh field of 16.4T.Conclusion
We demonstrated the ability to detect high-quality action potentials inside the bore of a 16.4T horizontal-bore animal scanner despite largely increased common mode noise compared to recordings made outside the scanner bore. An adaptive virtual referencing approach was developed to reduce the common mode noise and uncover action potentials that were in some cases completely obscured by noise. This technique shows promise for performing simultaneous fMRI and extracellular recording of action potentials at 16.4T to further study neurovascular coupling in animal models under activated or resting state.Acknowledgements
This work was supported in part by NIH grant R01 MH111413, S10 RR025031, NIBIB P41 EB027061, P30 NS076408, and by the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative.References
1. Ogawa S, Lee T-M, Nayak AS, et al. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 1990;14(1):68–78.
2. Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys. J. 1993;64(3):803-812.
3. Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage 2000;12(2):230–239.
4. Bonmassar G, Purdon PL, Jääskeläinen IP, et al. Motion and ballistocardiogram artifact removal for interleaved recording of EEG and EPs during MRI. Neuroimage 2002;16(4):1127–1141.
5. Niazy RK, Beckmann CF, Iannetti GD, et al. Removal of FMRI environment artifacts from EEG data using optimal basis sets. Neuroimage 2005;28(3):720–737.
6. Hermans K, De Munck JC, Verdaasdonk R, et al. Effectiveness of Reference Signal-Based Methods for Removal of EEG Artifacts Due to Subtle Movements during fMRI Scanning. IEEE Trans. Biomed. Eng. 2016;63(12):2638–2646.
7. Krishnaswamy P, Bonmassar G, Poulsen C, et al. Reference-free removal of EEG-fMRI ballistocardiogram artifacts with harmonic regression. Neuroimage 2016;128:398–412.
8. Rey HG, Pedreira C, Quian Quiroga R. Past, present and future of spike sorting techniques. Brain Res. Bull. 2015;119:106–117.
9. Kuo SM, Morgan DR. Active noise control systems. New York: Wiley; 1996.
10. Idiyatullin D, Zhu W, Zhang Y, et al. Novel multi-slab GRASE sequences for fMRI. Comparison with EPI. In: Proc Intl Soc Mag Reson Med. Paris, France; 2018. p. 5457.
Figures