1340
Deciphering the contribution of extracellular glutamate and intracellular calcium signaling to the BOLD fMRI signal
Yuanyuan Jiang1, Xuming Chen2, Patricia Pais Roldán2, Bruce Rosen1, and Xin Yu1,2
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Cambridge, MA, United States, 2Max Planck Institute for Biological Cybernetics, Tuebingen, Germany
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Cambridge, MA, United States, 2Max Planck Institute for Biological Cybernetics, Tuebingen, Germany
Synopsis
We established a multi-modal fMRI platform with two-channel fiber optic recording based on a genetically encoded fluorescent reporter, iGluSnFR, for extracellular glutamate (Glu) sensing and intracellular calcium indicator, GCaMP6f. Different from the intracellular neuronal and astrocytic calcium transients, the Glu signal, peak responses of spikes and baseline drift, show unique correlation features to the BOLD fMRI signal. Here, we applied the multi-modal fMRI platform to decipher the cellular and molecular interaction underlying the BOLD fMRI signal through the neuro-glio-vascular network in animal brains.
Introduction
Here, we expressed genetically encoded fluorescent reporter iGluSnFR1 for extracellular glutamate (Glu) sensing and genetically encoded calcium indicator GCaMP6f for calcium sensing in both neurons and astrocytes, and applied two-channel fiber optic recording system in combination with BOLD fMRI2,3,4. The introduction of glutamate, a primary excitatory neurotransmitter, provides us key neuro-glio-vascular linkage for signal propagation in trans- and extrasynaptic transmission in synaptic release and astrocytic cycling, as well as hemodynamic coupling. Besides its rapid spiking temporal dynamics upon electrical stimulation in comparison to evoked neuronal or astrocytic calcium transients, the extracellular Glu baseline drift demonstrates a unique correlation pattern with the varied BOLD signal changes in the animal brain, indicating a Glu-mediated regulatory mechanism underlying the BOLD fMRI signal fluctuation upon sensory stimulation.Methods
All images were acquired with a 14.1 T/26cm horizontal bore magnet (Magnex), interfaced with an AVANCE III console (Bruker) and equipped with a 12 cm gradient set, capable of providing 100 G/cm with a rise time of 150 us (Resonance Research), at MPI. A transreceiver surface coil was used to acquire fMRI images and was optimized on phantom using the 14.1T scanner with an adjusted siemens console at Martinos center. The fMRI scans with block design were performed using 3D Echo planar imaging sequence: TR, 1.5 s, TE,11.5 ms, 1.92X1.92X1.92 cm3, FOV, 48X48X48 matrix, 400X400X400 μm3 spatial resolution. The reporter iGluSnFR and GCaMP6f were expressed by AAV5 virus in the two hemispheres forepaw somatosensory cortex (FP-S1) with Syn or GFAP promoter. Fiber optic (200 μm) was inserted into the area which expressed the cortex for fluorescent signal recording.Results
We first acquired evoked neuronal calcium and Glu signals with simultaneous fMRI from the FP-S1 of two hemispheres, respectively. Fig. 1A shows the fMRI maps and the time course of BOLD signals, which is simultaneously acquired with neuronal calcium and Glu responses. The iGluSnFR-based Glu spikes showed more rapid temporal dynamics than the GCaMP-based neuronal calcium transients upon stimulation(Fig. 1A). Besides the neuronal calcium, the evoked astrocytic calcium transients and Glu spikes were acquired with fMRI simultaneously to provide an overall view of the neuro-glial-vascular interaction with the multi-modal fMRI platform (Fig. 1 B). Interestingly, we observed the baseline drift of the Glu signal during the stimulation. Different from the intracellular neuronal Ca2+ responses upon stimulation(Fig. 2A), the Glu baseline drift signal is increased proportionally upon the stimulation frequency (Fig. 2A) and duration (Fig. 2B). There are distinct baseline drift dynamic features between the extracellular Glu and intracellular neuronal calcium signal in comparison with the concurrent BOLD fMRI signal at different stimulation frequency and duration(Fig. 2C), indicating that the Glu baseline drifts are not due to BOLD effects.Both evoked Glu spike and drift, neuronal and astrocytic Ca2+ transient amplitudes were increased proportionally to the stimulation intensity (Fig. 3A). However, compared with neuronal or astrocytic spike, Glu spike and baseline drift amplitudes show a stronger correlation to BOLD fMRI responses in averaged group data (Fig. 3B). Moreover, the trial-specific variation shows a strong correlation with Glu baseline drift in the same animal under the same stimulation paradigm due to brain state fluctuation(Fig. 3C). The distribution of BOLD vs.Glu fitting R value distribution histogram shows a better dependency of BOLD fMRI variance on the Glu baseline drift (Fig 3D). The results indicate that the extracellular Glu baseline may directly contribute to the fMRI signal based on the varied physiological brain state. Future studies will focus on elucidating the extracellular Glu clearance with astrocytic calcium signaling contribution to the varied fMRI signals at varied brain states.
Conclusion
Concurrent recordings of extracellular glutamate and intracellular neuronal calcium with BOLD fMRI make it possible to specify individual neurovascular signaling events at both cellular and molecular levels and better understanding the neurovascular coupling mechanism underlying BOLD fMRI signal.Acknowledgements
This research was supported by NIH Brain Initiative funding (RF1NS113278-01), and the S10 instrument grant (S10 RR023009-01) to Martinos Center, German Research Foundation (DFG) Yu215/3-1, BMBF 01GQ1702, and the internal funding from Max Planck Society.References
- Marvin, J. S., Borghuis, B. G., Tian, L., Cichon, J., Harnett, M. T., et al. (2013). An optimized fluorescent probe for visualizing glutamate neurotransmission. Nature methods, 10(2), 162.
- Wang, M., He, Y., Sejnowski, T. J., and Yu, X. (2018). Brain-state dependent astrocytic Ca(2 +) signals are coupled to both positive and negative BOLD-fMRI signals. Proc. Natl. Acad. Sci. U.S.A. 115, E1647–E1656. doi: 10.1073/pnas.1711692115.
- He, Y., Wang, M., Chen, X., Pohmann, R., Polimeni, J. R., Scheffler, K., et al. (2018). Ultra-Slow Single-Vessel BOLD and CBV-Based fMRI spatiotemporal dynamics and their correlation with neuronal intracellular calcium signals. Neuron 97, 925–939.e5. doi: 10.1016/j.neuron.2018.01.025
- Chen, Y., Pais-Roldan, P., Chen, X., Frosz, M. H., & Yu, X. (2019). MRI-guided robotic arm drives optogenetic fMRI with concurrent Ca 2+ recording. Nature communications, 10(1), 2536.
Figures
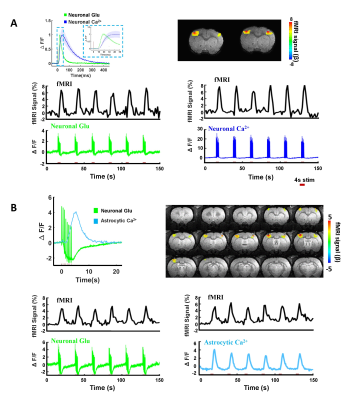
Figure 1. Characterizations of neuronal Glu, neuronal Ca2+, astrocytic responses
and BOLD signal in rat somatosensory cortex by evoked forepaw electrical
stimulation. (A) The BOLD fMRI signal and representative time course of
neuronal Glu and neuronal Ca2+. (B) The BOLD fMRI signal and representative time
course of neuronal Glu and astrocytic Ca2+ expressed in rat somatosensory cortex by evoked forepaw electrical
stimulations (2Hz, 4s).
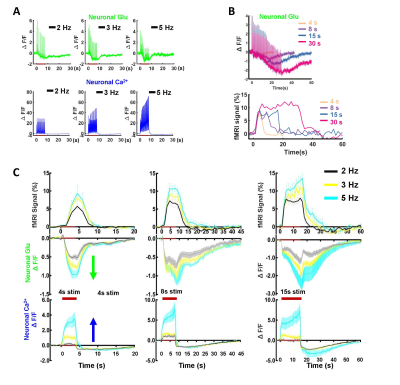
Figure 2.Observation and characterization of distinct baseline
drift kinetics for neuronal Glu and neuronal Ca2+ upon stimulation. (A) The representative stimulation frequency-dependent
neuronal Glu, neuronal Ca2+ (2, 3, 5Hz, 8s stim). (B) The
representative stimulation duration-dependent Glu baseline drift and BOLD
signal(4, 8, 15, 30s, 2Hz stim). (C) The comparison of Glu drifts shape,
neuronal Ca2+ baseline shape, and BOLD signal at different stimulation
frequencies and duration (2, 3, 5 Hz with a duration of 4, 8, 15s, 2Hz, n=7).
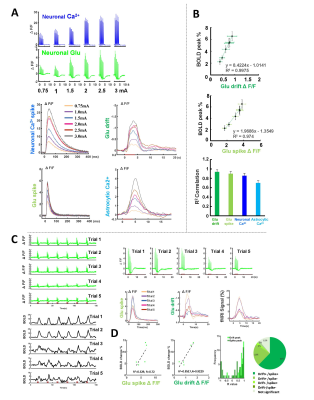
Figure 3. The linkage
of neuronal Glu baseline drift with the BOLD signal. (A) The representative amplitude-dependent of Glu spikes, Glu baseline drift, neuronal Ca2+, and astrocytic Ca2+
signal (0.75-3 mA, 2Hz 4s). (B) The correlation of those signal peaks with BOLD peaks at different amplitudes. (C) The representative trial-to-trial Glu signal and BOLD fMRI
signal variation from one subject. (D) The brain state dependence fitting R2 for the Glu spikes and drift peak with BOLD signal
and coefficient R-squared fitting distribution at different trials (14 animals, 200 trials).