1338
Quantitative MRI and serum biomarkers detect acute and chronic vascular effects of e-cigarette use
Alessandra Caporale1, Shampa Chatterjee2, Michael C Langham1, Wensheng Guo3, Frank Leone4, Andrew Strasser5, and Felix W Wehrli1
1Radiology, Laboratory for Structural, Physiologic and Functional Imaging, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 2Physiology, Institute for Environmental Medicine, Perelman School of Medicine, Philadelphia, PA, United States, 3Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, PA, United States, 4University of Pennsylvania Medical Center, Pulmonary, Allergy & Critical Care Division, Philadelphia, PA, United States, 5Psychiatry, Center for Interdisciplinary Research on Nicotine Addiction, Philadelphia, PA, United States
1Radiology, Laboratory for Structural, Physiologic and Functional Imaging, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 2Physiology, Institute for Environmental Medicine, Perelman School of Medicine, Philadelphia, PA, United States, 3Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, PA, United States, 4University of Pennsylvania Medical Center, Pulmonary, Allergy & Critical Care Division, Philadelphia, PA, United States, 5Psychiatry, Center for Interdisciplinary Research on Nicotine Addiction, Philadelphia, PA, United States
Synopsis
The vascular effects of e-cigarette use were investigated in young adults (19-35 years). Blood draws and 3T-MRI data were collected from seven e-cigarette users, seven smokers, thirty nonsmokers, the latter replicating the measurements after one nicotine-free e-cigarette vaping session. MRI-protocol measured peripheral vascular reactivity in response to cuff-induced ischemia, quantifying femoral artery luminal flow mediated dilation (FMDL), blood flow velocity, venous saturation (SvO2). FMDL decreased by 33% acutely after vaping, consistent with 20% NOx reduction and elevated inflammation (C-reactive protein increased by 95%). Reactive hyperemia was blunted as a chronic effect of both smoking and vaping, paired with anomalous biomarkers.
Introduction
Electronic cigarette (e-cig) use, or vaping, has increased alarmingly among teenagers, with one vaper in five high school students, according to recent surveys[1]. Rather than being harmless water vapor, e-cig aerosol contains heavy metals, ultra-fine particles, free radicals, toxicants and carcinogens[2-4], and when inhaled in the presence of nicotine, it is known to cause acute endothelial dysfunction[5]. Previously, we investigated whether e-cig aerosol inhalation, in the absence of nicotine, had acute and detrimental effects on the vascular endothelium, and we were able to quantify these effects using a battery of quantitative MRI parameters (qMRI)[6]. Briefly, in nonsmokers after a single episode of vaping, peripheral vascular reactivity (PVR) was significantly impaired in the femoral vessels, with reduced arterial blood flow velocity (BFV) peak and upslope during reactive hyperemia, decreased luminal flow mediated dilation, and a drop in venous oxygen saturation (SvO2)[6]. In this work, we examined the relation between qMRI and biomarkers of oxidative stress and inflammation extracted from the blood serum of non-smokers, after acute exposure to non-nicotinized e-cig aerosol. In addition, we compared PVR measurements and serum markers between the non-smokers group and a small group of newly recruited smokers and e-cig users, to observe the effect of chronic exposure to e-cig aerosol or conventional smoke on the vascular endothelium.Methods
Single users of either conventional cigarette (smokers) or e-cig (vapers), with a history of smoking/vaping of at least one year (Figure 1A) were recruited. Smokers and e-cig users underwent a single blood draw followed by a three-step MRI protocol (Figure 1B-D) performed at 3T (Siemens Prisma). Non-smokers only were subjected to an e-cig challenge entailing vaping a nicotine-free e-cig (16 puffs, 3s each; qMRI reported in [6]). The present study encompasses results of the first part of the MRI protocol only, quantifying PVR to cuff-induced ischemia. A sphygmomanometer cuff (Hokanson, Bellevue, Wash) was placed proximal to the superficial femoral vessels. After a baseline period (2 min) the cuff was inflated quickly for 5 min to 210–220 mmHg, allowing 5 min of recovery, post-deflation (Figure 2A). An eight-channel extremity transmitter-receiver coil (Invivo, Pewaukee, Wis) was used for the MRI scans (sequence details in [6]). The red and blue blocks in Figure 2B-C represent the time-resolved acquisition of blood flow velocity (BFV), and venous oxygen saturation (SvO2) measurement via MRI susceptometry[7], at baseline. Immediately after cuff release, BFV and SvO2 were resolved dynamically during the same TR cycle, at reactive hyperemia (Figure 2D). At rest and additional three time points post cuff release the arterial lumen was measured with high resolution rapid vessel-wall imaging (Figure 2E) to quantify luminal flow mediated dilation (FMDL) as the fractional change in arterial lumen. Concentrations of nitric oxide metabolites (NOx) and C-reactive protein (CRP), markers of oxidative stress and inflammation, respectively, were measured via ELISA and colorimetric assays of serum. Post vs pre-e-cig vaping differences in the serum biomarkers and qMRI were evaluated with Hotelling’s T2 test, together with Pearson’s correlation coefficients between qMRI and serum biomarkers. Differences between the three groups of non-smokers (before e-cig vaping), smokers and vapers were assessed by means of Dunnett’s test, considering non-smokers as the comparison group.Results and Discussion
Serum markers and most of the qMRI-metrics of PVR showed significant alterations post vs pre (nicotine-free) e-cig vaping (Figure 3). FMDL was impaired by over 30% (P<0.0001), together with NOx reduction (-20%, from 35.3 to 28.2 µmol/L; P<0.005). This could result from enhanced reactive oxygen species formation upon exposure of the vascular endothelium to nicotine-free e-cig aerosol[8], limiting NO bioavailability. There was large inter-subject variability of the inflammatory burden in the circulating serum, expressed by CRP. Pre-vaping, non-smokers with lower CRP levels showed larger FMDL, consistent with prior findings[9]. Post-vaping, CRP almost doubled (+95%, from 428.6 to 835.6 ng/ml; P<0.005), and measures of dynamic oxygen saturation (SvO2) were altered, including lower pre-cuff SvO2b, increased overshoot and shorter washout time (Tw). Reactive hyperemia was blunted acutely in nonsmokers post-vaping, as indicated by reduced peak velocity (VP), upslope (HI), and area under the hyperemic curve, (AUCa); reduced VP and HI were found also in e-cig vapers, as well as smokers (Figure 4), the latter confirming previous findings[7]. Preliminary results of comparison among the three groups (Figure 5), show that NOx bioavailability was significantly reduced after chronic exposure to e-cig aerosol (with nicotine). Systemic inflammation was higher in smokers, with CRP levels comparable between vapers and the nonsmokers’ group. In distinction, RI was increased similarly in smokers and vapers, suggesting greater vascular resistance, which may be due to increased blood viscosity. Reactive hyperemia AUC was significantly lower in smokers than in non-smokers (P<0.05) and trended towards lower values in vapers, as well as did hyperemic peak (VP).Conclusions
E-cig inhalation elicits acute endothelial dysfunction in healthy nonsmoking adults and triggers an inflammatory response, even in the absence of nicotine. Chronic exposure to e-cig aerosol for more than one year seems to reduce the bioavailability of NO, increase vascular resistance, and to alter peripheral vascular reactivity, comparably to tobacco smoking, albeit without increasing noticeably serum CRP levels. The results in smokers and vapers need to be interpreted with caution given the limited sample size.Acknowledgements
This study was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL109545, R01HL139358).References
[1]. Cullen KA et al., Morb Mortal Wkly Rep 2018; 67(45):1276–1277. [2]. Wang P et al., PLoS One 2017;12(1):e0169811. [3]. Goel R et al., Chem Res Toxicol 2015;28(9):1675–1677. [4]. Williams M et al., PLoS One 2013;8(3):e57987. [5]. Carnevale R et al., Chest 2016;150(3):606–612. [6]. Caporale A et al., Radiology 2019;293(1):97-106. [7]. Langham MC et al., Journal of Cardiovascular Magnetic Resonance. 2015;17(1):19. [8]. Chatterjee S et al., Am J Physiol-Lung Cell and Mol Physiol. 2019;317(2):L155-66. [9]. Kovacs I et al., Eur J Heart Failure 2006;8(5):451-9.Figures
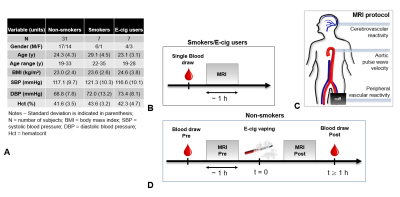
Figure 1. Study protocol. A. Smokers and
vapers underwent a single blood draw, immediately prior to the 50-min MRI protocol. B.
For non-smokers/non-vapers two blood samples were
collected. The same MRI protocol was performed twice, once before and once after a 5-min e-cigarette challenge. C. The MRI protocol
included a measure of peripheral vascular reactivity to cuff-induced ischemia,
followed by aortic pulse wave velocity quantification and cerebrovascular
reactivity assessment.
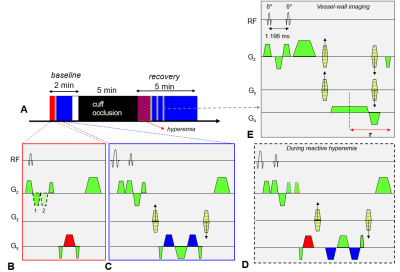
Figure 2. Peripheral vascular reactivity. A. Cuff-occlusion protocol. Red block represents acquisition of 1D-velocity projections (with two flow
encoding schemes in B). Blue block entails flow compensation for
phase difference mapping (C), yielding venous oxygen saturation (SvO2).
During reactive hyperemia, blood flow velocity (BFV) and SvO2 are resolved dynamically in
the same TR (D). At rest and at t=60, 90 and 120s the artery lumen is
measured using a 3D-water selective SSFP-echo sequence (E).
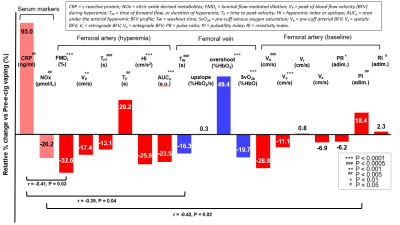
Figure 3. Relative change in serum markers and
MRI parameters, post vs pre-e-cig vaping. The serum markers are shown in
pink, the parameters related to arterial BFV and SvO2 are shown in red and blue, respectively. For each parameter, the level of significance P of the pre-post
association is provided (see legend). Significant linear correlations
between biomarkers and MRI parameters pre e-cig vaping are indicated below the
bar chart.
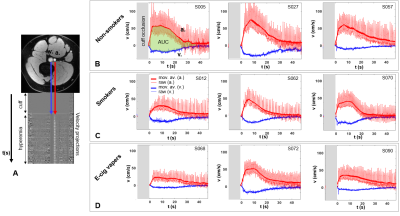
Figure 4. Reactive hyperemia. A. Axial slice of the thigh showing
femoral artery (a.) and vein (v.). The arrows point at 1D-velocity projections,
which give rise to the hyperemic profiles plotted in B-D. B-D. Blood flow velocity in a. (red)
and v. (blue) as a function of time. Moving average filter is applied (thick line). The subject ID number is indicated. AUC = area under the curve.
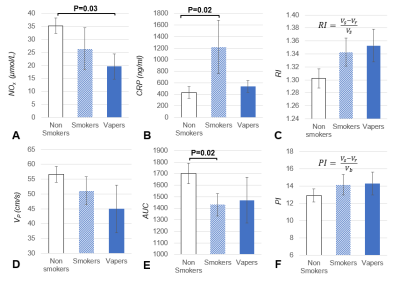
Figure 5. Serum markers and quantitative MRI parameters
in the three groups studies. Changes
in the serum markers and MRI parameters in smokers and e-cig users vs
non-smokers. A. Nitric oxide derived metabolites (NOx); B.
C-reactive protein (CRP); C. Resistivity Index (RI); D. Peak
arterial velocity during hyperemia (VP). E. Area under the
curve in the hyperemic arterial blood velocity profile (AUC). F.
Pulsatility index (PI). The significance level P is indicated when P<0.05.