1335
Screening Efficacy of Wearable Seismocardiography to Recommend MRI for Stratification of Aortic Valve Diseases1Biomedical Engineering, Northwestern University, Evanston, IL, United States, 2Anesthesiology, Northwestern University, Chicago, IL, United States, 3Radiology and Biomedical Engineering, University of Colorado, Anschutz Medical Campus, Aurora, CO, United States, 4Radiology, Northwestern, Chicago, IL, United States
Synopsis
Aortic valve diseases (AVD) require regular monitoring of aortic size and blood speeds. MRI can quantify morphology and flow parameters, such as velocity and wall shear stress, with high accuracy and reproducibility, especially as compared to echocardiography. However, the value proposition may be low for performing repeated MRI if there has been no change in disease state. A quick, inexpensive and easy to use test that identifies potential need for MR imaging could significantly raise the cost-effectiveness of using MRI for monitoring AVD. Here we show high potential screening efficacy of using seismocardiography to select AVD patients needing MRI examination.
Introduction
MR imaging techniques, such as real-time three-dimensional phase contrast '4D Flow' MRI, can create detailed portraits of cardiac and thoracic aorta flow function, including quantification of blood velocity and aortic wall shear stress. [1] The quantitative velocity measurements from 4D Flow are accurate and reproducible. [2–4] Clinically, this is particularly valuable in the context of managing aortic valve diseases (AVD), e.g., bicuspid aortic valve (BAV), which requires regular monitoring of blood velocities and aortic size to assess need for surgical intervention. [3–5] However, MR exams can be costly and time-intensive, so the value proposition is low for imaging frequently if there has been no change in disease state. For this reason echocardiography is more common in management of AVD. [5]A complementary measurement, the seismocardiogram (SCG) signal, is a quantification of physical vibrations propagating through the chest that are created by heart beats and pulsatile blood flow through the aorta. [6] It is inexpensive and easy to acquire, and it has been shown to contain meaningful information about a range of cardiac function parameters, although in less expansive detail than is accessible with MRI. Crucially, though, some SCG signal characteristics have been shown to correspond with chaotic, pathological aortic flow in patients with AVD. [7] Here, we evaluate potential screening efficacy of using a simple, single parameter from SCG to recommend for comprehensive MRI examination only in the cases where imaging is needed to resolve details of abnormal flow. The pairing of SCG with MRI can potentially raise the clinical value proposition of MRI in monitoring AVD and improve the standard of care for patients with such disease.
Methods
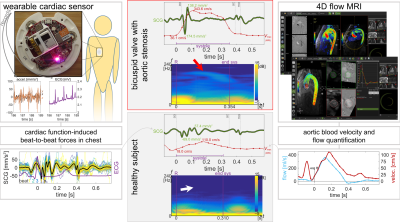
Data Collection: 39 healthy control subjects (50$$$\pm$$$18years, 15 female) and 8 patients (63$$$\pm$$$17years, 2 female) with aortic valve disease history and known flow abnormalities were recruited to an IRB-approved study. SCG signal acquisition was performed with subjects supine, immediately prior to 4D Flow MRI. SCG signals were acquired by a custom-built wearable with an accelerometer and ECG analog-to-digital converter (Fig.1). MRI was performed at 1.5T with imaging field-of-view covering the thoracic aorta; 2.4mm$$$^{3}$$$, 23frames/heartbeat resolution; and 150–375cm/s VENC set as appropriate from a flow scout image.Data Processing: Custom computer code was used to beat-align SCG signals and compute spectrograms for calculating RMS energy in the 120–240Hz range over the second half of systole for each subject. MR phase images were analyzed with commercial software to calculate blood velocity in a cutplane of the ascending aorta with manually-defined frame-by-frame regions-of-interest. Background phase correction and noise thresholding were performed in a semi-automated fashion by the software. Pathline visualizations were also generated inside a 3D aorta segmentation.
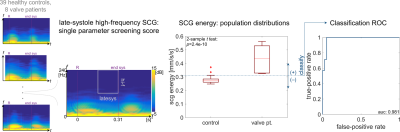
Statistical Analysis: Screening efficacy of the SCG energy metric was retrospectively assessed from all acquired MRI and SCG data. A two-sample $$$t$$$-test was used to compare energy level distributions between healthy controls and valve patients (Fig.2). Receiver-operator characteristic (ROC) analysis was performed, calculating the rates at which a thresholded-SCG energy classification would correctly or incorrectly recommend for MRI.
Results
Healthy subjects had coherent flow pathlines through the aortic arch and ascending aorta peak velocities of 105$$$\pm$$$21cm/s (cohort mean$$$\pm$$$standard deviation). All valve disease subjects had some flow abnormality as visualized by pathlines, such as jetting flow near the valve or swirling through the arch. Peak ascending aorta velocities for valve patients were 182$$$\pm$$$75cm/s. The RMS late-systole high-frequency SCG energy for healthy controls was significantly different than that of valve patients in a two-sample $$$t$$$-test, with $$$p\lt3\times10^{-10}$$$ (Fig.2). Thresholding SCG energy to classify need or no need for MRI examination correctly recommends MR for valve patients with a high true-positive rate and low false-positive rate (Fig.2). The ROC for this classification has area-under-curve (AUC) 0.98 (Fig.2). MRI showed normal flow function for healthy controls with coherent flow pathlines and non-pathological peak velocities, and it showed pathologically elevated peak velocities with jetting flow pathlines in valve disease patients (Figs.1,3).Discussion
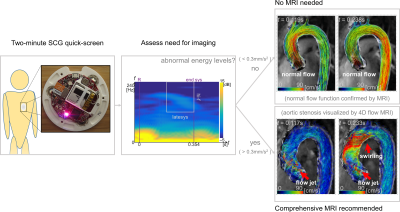
The potential screening efficacy of using SCG to identify need for MRI in patients with AVD was found to be high, with SCG-based disease state classification AUC above 0.95. This performance is comparable to more complex machine-learning classification techniques applied to other noninvasive measurements such as phonocardiography, but this classification method is a simple threshold of a single linear SCG signal feature. For clinical implementation, choice of threshold would proceed from an analysis in costs of unnecessary MRI balanced against need for positively identifying disease progression. The material costs for a wearable SCG sensor are around 100USD, its use requires virtually no training, and complete signal acquisition and analysis is possible in under two minutes.Conclusion
The high screening efficacy of SCG to identify aortic flow abnormalities can be used to raise the value proposition of employing MRI in clinical management of AVD. In such management, the need for regular monitoring of aortic size and blood velocities can make use of MRI unpalatable because imaging costs accrue, and the monitoring standard is echocardiography despite the comparative technical benefits of MRI. By pairing a simple, inexpensive SCG screen with high quality, expansively detailed 4D Flow MRI, it may be possible to make MR imaging accessible as a standard of care in AVD clinical management.Acknowledgements
This work was supported by the NIH and Hartwell Foundation.References
[1] Markl M, Schnell S, Barker AJ, 2014. "4D flow imaging: current status to future clinical applications". Current cardiology reports, 16(5), p.481.
[2] Markl M, Wallis W, Harloff A, 2011. "Reproducibility of flow and wall shear stress analysis using flow-sensitive four-dimensional MRI." Journal of Magnetic Resonance Imaging, 33(4), pp.988-994.
[3] Hundley WG, et al, 2010. "ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents". Journal of the American College of Cardiology, 55(23), pp.2614-2662.
[4] Zoghbi WA, et al, 2017. "Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance". Journal of the American Society of Echocardiography, 30(4), pp.303-371.
[5] Nishimura RA, et al, 2014. "2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines". Journal of the American College of Cardiology, 63(22), pp.e57-e185.
[6] Inan OT, et al, 2015. "Ballistocardiography and seismocardiography: a review of recent advances." IEEE J. Biomedical and Health Informatics 19(4), pp.1414-1427.
[7] Johnson EMI, et al, 2019. "Seismocardiography and 4D flow MRI reveal impact of aortic valve replacement on chest acceleration and aortic hemodynamics." Journal of Cardiac Surgery.
Figures