1331
Extracting the respiratory signal from Pilot Tone for highly accelerated, respiratory-resolved whole-heart 4D flow imaging1Biomedical Engineering, The Ohio State University, Columbus, OH, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Dorothy M. Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH, United States, 4Siemens Medical Solutions USA, Inc., Columbus, OH, United States, 5Cardiovascular Medicine and Radiology, The Ohio State University, Columbus, OH, United States
Synopsis
Pilot Tone has recently been proposed as a novel approach towards physiological signal monitoring. Unlike self-gating, often relied upon by free-running, respiratory-resolved imaging sequences, Pilot Tone is generalizable to a multitude of imaging techniques without requiring additional pulse sequence modification or specialized k-space sampling. In this work, we combine Pilot Tone with our previously described highly accelerated and fully self-gated whole-heart 4D flow framework to reconstruct respiratory-resolved 4D flow images in three healthy subjects. We compare Pilot Tone and self-gating derived respiratory binning and demonstrate good agreement in aortic and pulmonary artery flow quantification between the two methods.
INTRODUCTION
Contemporary advancements in free-running, respiratory resolved imaging sequences have primarily relied on self-gating (SG) for physiological signal monitoring.1,2,3 Recently, the novel Pilot Tone (PT) technique4 has been proposed which, unlike SG, is generalizable to a multitude of pulse sequences without additional sequence modification to acquire self-gating data. In this work, we combine PT with our previously described highly accelerated and fully self-gated whole-heart 4D flow framework5 to reconstruct respiratory-resolved 4D flow images and compare the impact of SG versus PT derived respiratory binning on flow quantification in the aorta and pulmonary arteries in 3 healthy subjects.METHODS
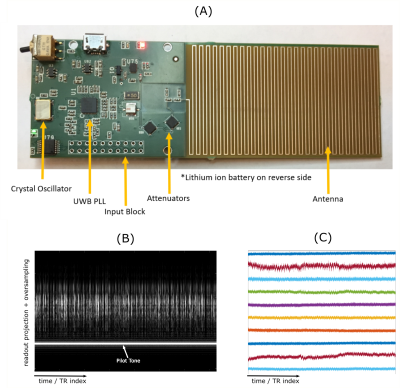
Three healthy subjects were prospectively recruited and scanned on a 1.5T clinical MR system (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany). A custom-built tunable, narrow-band RF source (Figure 1A) was secured upon the chest receiver coil array, providing continuous transmission of the PT.4,6 Subjects were scanned using a prototype, fully self-gated, Cartesian 4D flow sequence5 with sagittal slab prescription covering the whole heart. Acquisition parameters for 4D flow were as follows: spatial resolution = 2.6-3mm x 2.4-2.8mm x 2.4-2.8mm, FOV = 230-270mm x 310-360mm x 134-156mm, BW = 744 Hz/px, VENC = 150 cm/s, TE/TR = 2.6/4.7 ms, and FA = 7°. PT frequencies were tuned to reside in the oversampled readout region and transmitted simultaneously with acquisition. All 4D flow acquisitions were acquired with a fixed 5-minute duration.Interleaved superior-inferior (SI) projections acquired every 9th TR (~42 ms) were extracted from the SG 4D flow raw data and processed via temporal filtering followed by principal component analysis (PCA) to obtain cardiac and respiratory surrogate signals used for binning. The raw PT signal (Figures 1B-C), was similarly extracted and processed through temporal filtering, PCA, and independent component analysis. Transmitter hardware limitations only allowed reliable extraction of a respiratory PT signal. Data was binned into a matrix of 4 by 20 phases in respiratory and cardiac dimensions respectively, with respiratory bins weighted according to a soft-gating7 approach (Figure 2). Cardiac phases were always determined by SG while respiratory phases were determined by PT or SG. Respiratory bin sizes were chosen to contain an equal number of samples corresponding to an acceleration rate of 30. Reconstructions were performed for each respiratory bin using the previously described ReVEAL4D.8 Volumetric flow rate and net volumetric flow were computed in the ascending, arch, and descending aorta (Aao, Arch, Dao) as well as in the main, right, and left pulmonary arteries (MPA, RPA, LPA) for each subject at end-expiration (EE) and end-inspiration (EI) to compare the impact of flow quantification using data binned based on SG versus PT. The arch from one subject was not included due to insufficient coverage in the SI direction. Flow quantification was performed in the 4D Flow v2.4 software package (Siemens Healthcare, Erlangen, Germany). Correlation and Bland-Altman analysis were used to assess quantification agreement.
RESULTS
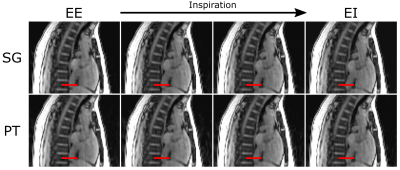
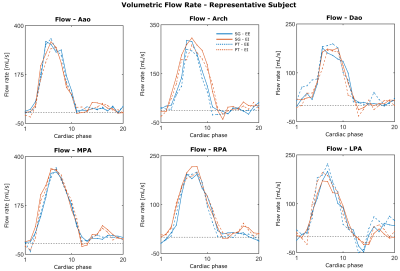
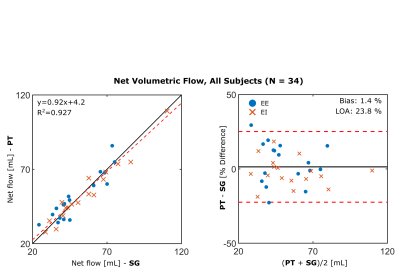
Figure 3 shows sagittal cross-sections from the 4 respiratory phases reconstructed from one subject. From EE to EI, there is noticeable respiratory motion, but for a given phase, respiratory states are well matched between SG and PT. Figure 4 depicts volumetric flow curves from the same subject, which, again, demonstrate similarity between the two signals. Among the 34 net flow measurements performed, there is a strong correlation (Figure 5) between the two binning methods (slope: 0.92, intercept: 4.2, R2: 0.927). Bland-Altman analysis reveals a non-significant bias (P=0.499) of 1.4% with limits of agreement (LOA) of 23.4%, reported in percent difference.CONCLUSIONS
This early data suggests that PT-based respiratory binning may provide similar performance and quantification as SG when coupled with highly accelerated 4D flow, making PT an attractive alternative to SG. Further work will explore the use of PT for both respiratory and cardiac binning and its impacts on flow quantification.Acknowledgements
This work was supported by NIH grant R21EB026657.References
1. Feng et al. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016;75(2):775-788.
2. Han et al. Self-gated 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) using rotating cartesian K-space (ROCK): Validation in children with congenital heart disease. Magn Reson Med. 2017;78(2):472-483.
3. Di Sopra et al. An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magn Reson Med. 2019;82(6):2118-2132.
4. Speier et al. PT-Nav: A Novel Respiratory Navigation Method for Continuous Acquisition Based on Modulation of a Pilot Tone in the MR-Receiver. In Proc. ESMRMB. 2015;129:97-98.
5. Pruitt et al. Self-gated 5-minute whole-heart 4D flow imaging. In Proc. 27th Annual ISMRM. 2019:1955.
6. Lenk et al. Respiratory Motion Tracking in Magnetic Resonance Imaging with Pilot Tone Technology. [master's thesis]. Columbus, OH: The Ohio State University; 2018.
7. Forman C, Piccini D, Grimm R, Hutter J, Hornegger J, Zenge MO. Reduction of respiratory motion artifacts for free-breathing whole-heart coronary MRA by weighted iterative reconstruction. Magn Reson Med. 2015;73(5):1885-1895. doi:10.1002/mrm.25321
8. Rich et al. A Bayesian approach for 4D flow imaging of aortic valve in a single breath-hold. Magn Reson Med. 2019;81:811-824.
Figures