1328
Self-gated dynamic contrast enhanced magnetic resonance imaging of the aortic root in atherosclerotic mice: a natural progression study1Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2University of Amsterdam, Amsterdam, Netherlands
Synopsis
Enhanced endothelial permeability is an important hallmark of atherosclerotic plaques at high-risk for causing severe cardiovascular events. Here we present a the application of a novel, self-gated DCE-MRI acquisition combined with compressed sensing reconstruction to quantify endothelial permeability in the mouse aortic root. In a longitudinal natural disease progression study in ApoE-/- mice, we find that plaque contrast agent washout computed from this acquisition changes significantly over time, with washout being slower in older mice.
Introduction
Cardiovascular disease due to atherosclerosis is the first cause of morbidity and mortality worldwide1. Vessel wall extravasation of inflammatory cells, facilitated by increased endothelial permeability, is crucial for the initiation and progression of atherosclerosis2. Much effort has been devoted to developing and validating in vivo quantitative imaging techniques to measure plaque permeability as a marker of vulnerable plaques, both in humans3-5 and in large animal models of atherosclerosis6,7. We present the development of in vivo dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) to quantify endothelial permeability in the atherosclerotic mouse aortic root. We apply and validate DCE-MRI in a longitudinal study using genetically modified atherosclerotic ApoE-/- mice, to investigate changes in aortic root endothelial permeability during natural disease progression, in comparison with histological measures of plaque inflammatory cell burden.Methods
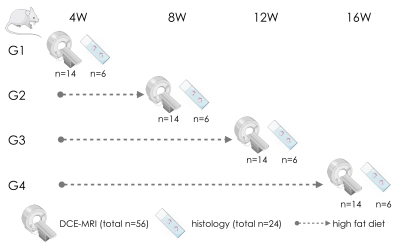
Study design. 32, 8 weeks old, female ApoE-/- mice were divided into 4 groups. All mice were fed a high cholesterol diet and were imaged at 4, 8, 12 and 16 weeks after diet initiation. A total of 24 mice was euthanized for ex vivo validation (Figure 1).Self-gated DCE-MRI. We used a self-gated fast low angle shot (FLASH) MRI acquisition combined with compressed sensing reconstruction for aortic root DCE MRI 8-10. This strategy enables high-resolution imaging, stable steady-state contrast, and simultaneous cardiac and respiratory motion compensation without relying on ECG and respiratory triggering. k-space lines acquired over consecutive heartbeats were binned into separate cardiac phases based on a navigator signal and into separate temporal dynamic frames before and after contrast agent injection. After the acquisition of scout images, self-gated DCE-MRI was performed for 32 minutes using a single slice, black blood FLASH acquisition, before, during and after the injection of 0.3 mmol/Kg of Gd-DTPA. Images were reconstructed with a non-linear compressed sensing algorithm with total variation regularization in the cardiac-cycle dimension. Inner and outer vessel wall contours were traced for all dynamic temporal frames, in the same phase of the cardiac cycle. Regions-of-interest (ROI) curves from the aortic root were extracted using in house software. Signal intensity from regions-of-interest (ROIs) enhancement curves was converted to pseudo-concentration values using a validated linear relationship, and then analyzed using a piecewise curve model11.
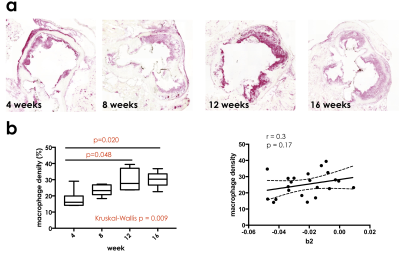
Histology. Histological sections were stained using CD68 antibodies to quantify plaque inflammatory cells (macrophages), using a previously validated protocol. Macrophage density was calculated as the percent ratio between macrophage rich areas and vessel wall area.
Statistical analysis. After appropriate tests for normality, changes in DCE-MRI parameters and macrophages density over time were evaluated using non parametric Kruskal-Wallis tests, and Spearman correlations.
Results
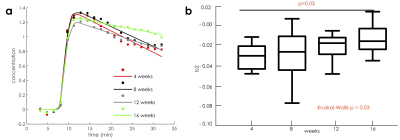
DCE-MRI. Contrast agent washout from the aortic root (represented by the modeling variable b2) was found to be significantly different among different cohorts of mice (Figure 2, p =0.03). Side-by-side comparison of groups in pairs revealed significant differences between mice on high fat diet for 4 versus 16 weeks (p=0.03). Spearman correlation also revealed a significant association between b2 and the number of weeks spent on high fat diet (p=0.003, r =0.41).Histology. Macrophage density significantly also increased in mice fed a high fat diet for longer periods of time (p = 0.009, Figure 3). Spearman correlation revealed a significant association between the number of weeks on high fat diet and macrophage density (p<0.001, r = 0.70). Between groups comparison revealed significant differences between mice fed a high fat diet for 4 versus 12 weeks (p = 0.048), and 4 versus 16 weeks (p=0.020), mirroring differences found in in vivo DCE-MRI parameter b2. Additionally, there was a positive trend in relationship between the parameter b2 from DCE-MRI and macrophage density for each mouse (r = 0.28, p=0.20).
Discussion
We describe the development and validation of self-gated DCE-MRI with compressed sensing reconstruction for the quantification of endothelial permeability in the mouse aortic root with high spatio/temporal resolution, and compare this measure to plaque accumulation of inflammatory cells. Other authors have applied quantitative contrast enhanced MR imaging methods to measure endothelial permeability in the atherosclerotic mouse vasculature, such as post-contrast R1 relaxation mapping, which, however might be partially dependent also on the contrast agent distribution volume. Our study suffers from several limitations. Firstly, while black blood imaging allows for better vessel wall delineation, it does not allow performing kinetic modeling using standard methods, since contrast agent concentration in the blood plasma cannot be reliably sampled. Lastly, our image acquisition encompassed only 1 slice, to allow for sufficiently high temporal resolution to capture contrast agent kinetics, together with adequate spatial resolution for imaging of the small mouse aortic root. However, this strategy may however render the comparison with histology or other ex vivo assays quite challenging.Conclusions
In conclusion, we present the development and validation of high spatio/temporal DCE-MRI to quantify changes in endothelial permeability in a mouse atherosclerosis model. Our results show that the contrast agent washout rate from atherosclerotic plaque is significantly different in mice kept on high fat diet for different lengths of time. Furthermore, we show that these changes are mirrored by similar changes in plaque inflammatory cell burden over time.Acknowledgements
No acknowledgement found.References
1 Benjamin, E. J.et al.Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation137, e67-e492, doi:10.1161/CIR.0000000000000558 (2018).
2 Gimbrone, M. A., Jr. & Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res118, 620-636, doi:10.1161/CIRCRESAHA.115.306301 (2016).
3 Kerwin, W.et al.Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation107, 851-856 (2003).
4 Kerwin, W. S.et al.Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology241, 459-468, doi:10.1148/radiol.2412051336 (2006).
5 Kerwin, W. S., Oikawa, M., Yuan, C., Jarvik, G. P. & Hatsukami, T. S. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med59, 507-514, doi:10.1002/mrm.21532 (2008).
6 Calcagno, C.et al.Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol28, 1311-1317, doi:10.1161/ATVBAHA.108.166173 (2008).
7 Calcagno, C.et al.Three-dimensional dynamic contrast-enhanced MRI for the accurate, extensive quantification of microvascular permeability in atherosclerotic plaques. NMR Biomed28, 1304-1314, doi:10.1002/nbm.3369 (2015).
8. Coolen, B. F.et al.High frame rate retrospectively triggered Cine MRI for assessment of murine diastolic function. Magn Reson Med69, 648-656, doi:10.1002/mrm.24287 (2013).
9 Coolen, B. F.et al.Three-dimensional T1 mapping of the mouse heart using variable flip angle steady-state MR imaging. NMR Biomed24, 154-162, doi:10.1002/nbm.1566 (2011).
10 Motaal, A. G.et al.Accelerated high-frame-rate mouse heart cine-MRI using compressed sensing reconstruction. NMR Biomed26, 451-457, doi:10.1002/nbm.2883 (2013).
11 Loerakker, S.et al.Ischemia-reperfusion injury in rat skeletal muscle assessed with T2-weighted and dynamic contrast-enhanced MRI. Magn Reson Med66, 528-537, doi:10.1002/mrm.22801 (2011).
Figures