1326
iSNAP sequence for simultaneous measurement of whole brain dynamic MRA, MRA, intracranial vessel wall and T1W structural brain MRI1Vascular Imaging Lab and BioMolecular Imaging Center, Radiology, University of Washington, Seattle, WA, United States, 2Philips Research North America, Cambridge, MA, United States, 3Biomedical Engineering, King's College London, London, United Kingdom, 4Surgery, University of Washington, Seattle, WA, United States
Synopsis
In this study, a whole brain sequence named iSNAP, with 0.8 mm isotropic voxel size and 6 min 30 sec acquisition time, was developed to simultaneously obtain four different image contrasts (dynamic MRA [dMRA] for blood flow monitoring, MRA for luminal stenosis measurement, black blood image for vessel wall [VW] measurement and T1W for brain structural imaging). Preliminary testing results on a healthy volunteer and two patients with cerebrovascular diseases demonstrated the feasibility and potential of the sequence.
Introduction
A comprehensive assessment of intracranial vascular conditions, including vessel lumen, wall, blood flow, and brain structure is required. However, it is time consuming to acquire all of these images with current MR imaging techniques. In this study, a highly time-efficient whole brain sequence named iSNAP was developed to simultaneously obtain four different image contrasts (dynamic MRA [dMRA] for blood flow monitoring, MRA for luminal stenosis measurement, black blood image for vessel wall [VW] measurement and T1W for brain structural imaging), and was evaluated in healthy volunteers and patients with known intracranial atherosclerosis.Methods
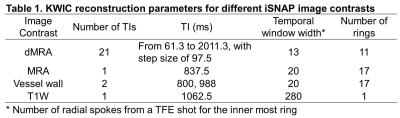
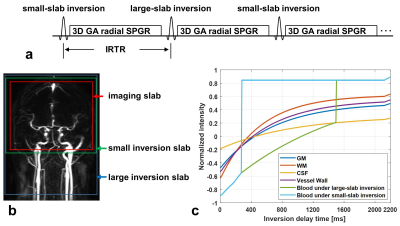
Sequence design and image reconstruction strategy: SNAP was an MR sequence for simultaneous imaging of MRA and intraplaque hemorrhage [1]. GOAL-SNAP was an extension of SNAP for carotid vessel wall T1 mapping by using inversion preparation and 3D golden angle radial spoiled gradient echo readout [2]. In GOAL-SNAP, KWIC method [3] was used to reconstruct multiple inversion delay time (TI) images, which were subsequently used to estimate T1 values. iSNAP is an extension of GOAL-SNAP for brain imaging by further adoption of FAIR-ASL preparation [4] (Figure 1a), i.e. the 3D acquisition was performed twice, one with a small-slab inversion, the other with a large-slab inversion (Figure 1b). The simulated signal evolution for different tissue is shown in Figure 1c. Then different strategies for raw data manipulation and image reconstruction were used to reconstruct images with different contrasts (reconstruction parameters are shown in Table 1). To obtain dMRA, images at multiple TIs were reconstructed using KWIC with small temporal window width (i.e. number of spokes from a shot for the inner most KWIC ring) and small number of rings, thus minimizing temporal blurring, for both inversion preparations. Then subtraction of the corresponding TI images between the two acquisitions was performed. To obtain a traditional MRA, ASL-subtraction was used as well, but the images were reconstructed at a single and intermediate TI using KWIC with a larger temporal window width and a larger number of rings, thus enabling the image to include most arteries and have higher SNR. The VW images were reconstructed from the acquisition with large-slab inversion preparation only, and the TIs were chosen such that the blood signal is around the zero-crossing point. KWIC with a larger temporal window width and a larger number of rings was adopted as well. To obtain T1W structural images, data from the two preparations were averaged to improve SNR before reconstruction with traditional non-uniform FFT. MR experiments: iSNAP was tested on healthy volunteers and patients with cerebrovascular disease using a Philips Ingenia 3T MR scanner (Philips Healthcare, Best, The Netherlands), equipped with a 32-ch head coil. For patient scans, we also acquired the original SNAP (with voxel size 0.8 mm isotropic), 3D TOF (with voxel size 0.5×0.5×1 mm3) and 3D black blood T1-VISTA (with voxel size 0.5 mm isotropic) vessel wall images. Local IRB approved the imaging protocol and informed consent was obtained from all participants. Imaging parameters for iSNAP were: Flip angle 6°, TR/TE 7.6/2.7 ms, voxel size 0.8mm isotropic, thickness of the small/large inversion slab 137.5/500mm, TFE factor 280, total number of TFE shots 186, shot interval (i.e. the IRTR in Figure 1a) 2125 ms, total scan time 6 min 35 sec.Results
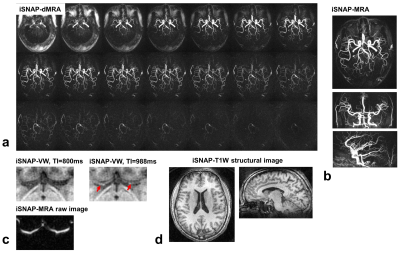
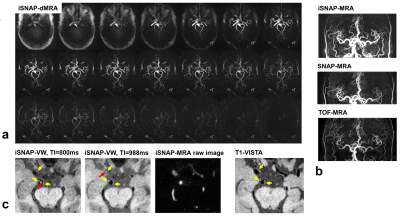
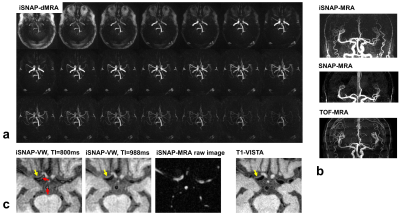
So far, one healthy volunteer and two patients were scanned. The KWIC reconstruction parameters for the different image contrasts were determined after performing some tuning tests (Table 1). Figure 2 shows the four different image contrasts obtained from iSNAP on the healthy volunteer, while Figure 3 and 4 show images of iSNAP, original SNAP, TOF and T1-VISTA from the two patients, respectively. Generally, robust and good image quality was achieved from iSNAP for dMRA, MRA and T1W structural imaging. The performance of blood suppression in the iSNAP-VW images shows variations over subjects and TIs. However, due to the multi-TI and multi-contrast capability of iSNAP, both lumen and vessel wall can still be separated and lesions can be identified.Discussion and Conclusion
Our preliminary results suggest that iSNAP can simultaneously provide MRA, dynamic MRA, vessel wall and brain structural assessment in a single scan with scan time around 6 min 30 sec. With additional optimization, iSNAP has potential to become a highly time-efficient and feasible solution for comprehensive assessment of cerebral vascular health. Similar to GOAL-SNAP [2], a quantitative T1 map can also be derived from iSNAP. Future work will address variation in blood suppression on the vessel wall image. The variation of blood suppression effect in iSNAP-VW may be related to the inflow velocity, because even with a large-slab inversion, the very high velocity uninverted spins may have reached the imaging locations at the selected TIs. The 0.8 mm resolution of iSNAP may not be sufficient for detecting early intracranial wall lesions, but iSNAP should have a very high sensitivity to intraplaque hemorrhage, which has a very low T1, due to the availability of strong T1 weighting image. In summary, the current iSNAP implementation can detect large plaques including those with intraplaque hemorrhage and detect any stenosis or blood flow alterations accompanying these lesions. Such a capability has not been demonstrated before and will enable comprehensive evaluation of ischemic stroke.Acknowledgements
This research is supported by grants from the National Institutes of Health (R01NS092207, R01HL103609).References
1. Wang, J., et al., Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med, 2013. 69(2): p. 337-45.
2. Qi, H., et al., Carotid Intraplaque Hemorrhage Imaging with Quantitative Vessel Wall T1 Mapping: Technical Development and Initial Experience. Radiology, 2018. 287(1): p. 276-284.
3. Song, H.K. and L. Dougherty, k-Space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magnetic Resonance in Medicine, 2000. 44(6): p. 825-832.
4. Kim, S.G., Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med, 1995. 34(3): p. 293-301.
Figures