1325
Highly accelerated vessel wall imaging using CAIPIRINHA accelerated SPACE and IFR-CS
Sen Jia1,2, Zhilang Qiu1,2, Lei Zhang2, Xin Liu2, Hairong Zheng2, and Dong Liang2
1University of Chinese Academy of Sciences, Shenzhen, China, 2Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
1University of Chinese Academy of Sciences, Shenzhen, China, 2Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Synopsis
Joint intracranial and carotid 3D vessel wall imaging (VWI) with an isotropic spatial resolution between 0.5-0.6 mm is promising in diagnosing arterial wall diseases but leads to clinically impractical scan time. CAIPIRINHA 3x3 undersampling with elliptical scanning is used to expedite the VWI by 11-fold without less sampling the high-frequency region of k-space. Iterative L1-ESPIRiT algorithm is employed for reconstruction with GRAPPA result as the initialization. The scheme of Iterative Feature Refinement is embedded into L1-ESPIRiT iteration to avoid potential over-smoothing issue. Finally, the 3D 11x VWI scan at an isotropic resolution of 0.6 mm takes only 3.5 minutes.
Introduction
High-resolution MR vessel wall imaging (VWI) is promising in detecting and characterizing wall diseases such as atherosclerosis plaque, which frequently occur in the intracranial and/or carotid vascular territories 1. Jointly performing intracranial and carotid VWI facilitates comprehensive and efficient examination of wall lesions in a single 3D scan 2. However, achieving high spatial resolution and whole brain and neck coverage simultaneously will lead to clinically impractical scan time. Long acquisition time would deteriorate the motion robustness of VWI on patients and induce intensive tradeoff between achievable imaging resolution and scan time.Methods
Combined Compressed Sensing and Parallel Imaging was utilized to highly expedite the 3D VWI scan. Instead of using the usual variable density Poisson-disc sampling (vdPDS), CAIPIRINHA 3 3x3 undersampling with elliptical scanning was utilized in this work. The equidistant sampling scheme avoids the less sampling of high-frequency region of k-space in vdPDS which is crucial to the image sharpness. Iterative L1-ESPIRiT algorithm 4 was used for reconstruction with a GRAPPA result as the initialization. GRAPPA initialization would benefit the suppression of aliasing artifacts and reduce the required iteration number to reach convergence. The Iterative Feature Refinement 5 (IFR) scheme was integrated into the iteration process of L1-ESPIRiT seamlessly to avoid the potential over-smoothing problem due to nonlinear filtering.In-vivo experiments were IRB approved with written informed consents obtained from all subjects. All VWI scans were performed on a 3T scanner (Siemens Tim Trio, Germany) with a 32-channel head and neck coil. Imaging parameters were: T1 weighted SPACE sequence with non-selective excitation and sagittal imaging orientation, FOV = 230-240 (RO, head-foot) x 210 (PE, anterior-posterior) x 192 (PAR, left-right) to achieve whole brain and neck coverage, encoding matrix size = 384-400 x 352 x 320, imaging resolution = 0.6 mm with 2x interpolated reconstruction, TE/TR = 10/1000 ms, echo train length (ETL) = 48. The scan time of 3D VWI of isotropic 0.6 mm resolution was 3.5 minutes. The CAIPIRINHA accelerated datasets were reconstructed online by GRAPPA and IFR-L1ESPIRiT algorithms respectively. Both were implemented in the open-sourced Gadgetron software 6. The GRAPPA reconstruction and the ESPIRiT calibration step of IFR-L1ESPIRiT run on two 40-cores CPUs with 256GB memory, while the iteration step run on three Nvidia Geforce 980Ti GPUs with 18GB memory. The computational time for IFR-L1ESPIRiT with 20 iterations was approximate 3 minutes.
Results
Figure 1 illustrates the VWI images obtained by the proposed 11-fold acceleration scheme on a 62-years old healthy male volunteer. Intracranial and carotid arterial walls are clearly depicted on the curved multiplanar reconstruction (CMPR) of the resulted 3D image. Blood is nulled by the inherent dark-blood capability of SPACE sequence. In Figure 2, the 11-fold CAIPIRINHA accelerated VWI is compared with 11x vdPDS accelerated VWI on a 60-years old healthy female volunteer. With the same reconstruction algorithm, the vertebral arterial walls are more clearly imaged on the CAIPIRINHA VWI image. The CAIPIRINHA accelerated dataset obtained from a 28-years old healthy male volunteer are reconstructed online by IFR-L1ESPIRiT and GRAPPA algorithms respectively. The results are compared in Figure 3. Both are free of aliasing artifacts, while the basilar vessel walls on the GRAPPA reconstruction are deteriorated by heavy noise amplification. The proposed fast VWI scheme alleviates the intense tradeoff between the imaging resolution and scan time. Figure 4 demonstrates the VWI results at isotropic 0.6-, 0.55- and 0.5-mm resolutions on a 27-years old healthy female volunteer. The three VWI scans are completed in 3.5, 4.2 and 5 minutes respectively. Improved isotropic resolution benefits the wall depiction but at a cost of visibly degraded signal-to-noise ratio due to the reduced voxel size.Discussion
Joint intracranial and carotid VWI acquired at an isotropic resolution of 0.6 mm was expedited into 3.5 minutes using 11-fold acceleration. CAIPIRINHA sampling and iterative feature refinement scheme were utilized together to improve the wall imaging quality. The proposed fast VWI could alleviate the intensive tradeoff between VWI imaging resolution and scan time.One limitation of proposed fast VWI method was the dramatically increased computational burden and reconstruction time due to the tremendous encoding matrix size. Further work will focus on boosting the reconstruction speed and improving the clinical robustness of proposed VWI technique.
Acknowledgements
This work is supported by the State Key Program of National Natural Science Foundation of China (Grant No. 81830056) and the National Natural Science Foundation of China (Grant No. 81801691).References
- Lindenholz A, van der Kolk AG, Zwanenburg JJM, Hendrikse J. The Use and Pitfalls of Intracranial Vessel Wall Imaging: How We Do It. Radiology 2018, 286:12-28.
- Jia S, Zhang L, Ren L, etc. Joint Intracranial and Carotid Vessel Wall Imaging in 5 minutes using Compressed Sensing accelerated DANTE-SPACE. European Radiology 2019, https://doi.org/10.1007/s00330-019-06366-7.
- Breuer FA, Blaimer M, Mueller MF, etc. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reason Med 2006, 55: 549-556.
- Uecker M, Lai P, Murphy MJ., etc. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med 2014, 71: 990-1001.
- Cheng J, Jia S, Ying L, etc. Improved parallel image reconstruction using feature refinement. Magn Reason Med 2018, 80: 211-223.
-
Hansen SM, Sorensen TS. Gadgetron: An Open
Source Framework for Medical Image Reconstruction. Magn Reson Med 2013,
69:1768-1776.
Figures
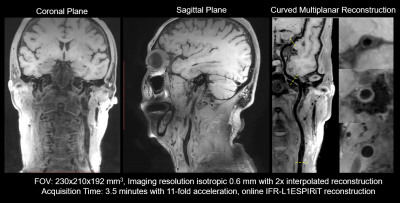
Figure 1. Joint intracranial and carotid
vessel wall imaging of isotropic 0.6 mm resolution could be scanned in 3.5 minutes by
11-fold Compressed Sensing acceleration.
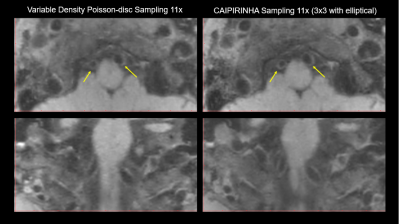
Figure 2. Comparing the wall imaging
quality between variable-density Poisson-disc sampling and CAIPIRINHA sampling
at 11-fold acceleration and the same L1-ESPIRiT reconstruction.
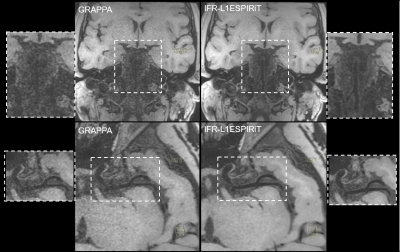
Figure 3. Comparing the wall imaging quality between parallel imaging only GRAPPA reconstruction and the proposed IFR-L1ESPIRiT reconstruction. The heavy noise
amplification by GRAPPA deteriorated the imaging quality of basilar arterial wall.
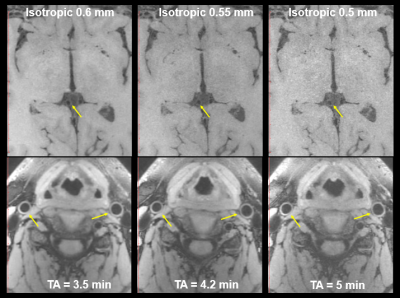
Figure 4. The proposed fast VWI
scheme alleviated the intense tradeoff between achievable imaging spatial resolution
and required scan time.