1319
Ultrashort TE Time-Spatial Labeling Inversion Pulse MRA for Simulated Visceral Arterial Diseases Indicated for Endovascular Interventions.1Department of Radiology, Tohoku University Hospital, Sendai, Japan, 2Diagnostic Radiology, Tohoku University Hospital, Sendai, Japan, 3Institute of Fluid Science, Tohoku University, Sendai, Japan, 4Canon Medical Systems corp., Tochigi, Japan
Synopsis
Visceral arterial diseases should be evaluated before and after endovascular interventions. We compared ultrashort TE (UTE) and steady-state free precession (SSFP) time-SLIP MRAs regarding their signal decay in pulsatile flow phantoms reflecting stenosis, aneurysm, and metallic stents. In all phantom models, UTE time-SLIP MRA provided superior visualization of target lumens to SSFP time-SLIP MRA. UTE time-SLIP MRA demonstrated minimal signal decay except for in-stent lumen of a stainless-steel stent. Our results indicated robustness of UTE time-SLIP MRA for intra-voxel spin dephasing caused by accelerated flow at the stenosis, turbulent flow in the aneurysm and susceptibility effects from metallic devices.
Introduction
Endovascular intervention using metal devices is indicated for most visceral artery diseases.1,2 Non-contrast time-spatial Labeling Inversion Pulse (time-SLIP) MRA using steady-state free precession (SSFP) technique is accepted for evaluating abdominal visceral arteries. Although SSFP technique provides high signal efficiency with intrinsic T2/T1 contrast, there are potentially degrading factors such as intra-voxel dephasing from accelerated blood flow in the stenosis, turbulent flow and susceptibility artifacts caused by metallic devices in the clinical settings (Fig.1). Ultrashort TE (UTE) based MRA was reported to provide better visualization of in-stent flow in intracranial arteries compared with conventional MRA.3,4 In this study, we aimed to evaluate the feasibility of UTE time-SLIP MRA for three types of pulsatile flow phantoms simulating visceral artery diseases before and after endovascular interventions by comparing with SSFP time-SLIP MRA.Methods
Phantom models (Fig.2).A) A renal artery stenosis phantom consisted of a 20mm-inner-diameter main trunk with 10 patterns of branches in 6mm diameter; each branch had either normal, 50% or 70% degree of concentric or eccentric area stenosis; each branch originated from the main trunk in the direction of either 45 or 90 degrees.
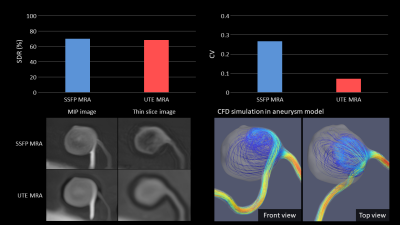
B) An aneurysm phantom modeled by 40mm-inner-diameter splenic artery aneurysm of a patient.
C) Three 6mm-inner-diameter tube phantoms with a stainless-steel stent (Palmatz Genesis, Cordis, Santa Clara, California), a nitinol stent-graft (Viabahn, W. L. Gore & Associates, Newark, Delaware), and a cobalt alloy multilayer flow modulator (Peripheral MFM, Cardiatis, Isnes, Belgium).
Image acquisition.
All phantoms were imaged with a 3T MR scanner (Vantage Titan 3T, Canon Medical Systems, Tochigi, Japan). UTE time-SLIP MRA was acquired with TR, 3.7ms, TE, 0.096ms, flip angle, 5 degrees, trajectory, 9960, segments, 120. SSFP time-SLIP MRA was acquired with TR, 4.8ms, TE, 2.4ms, flip angle, 80 degrees. Other common parameter settings were: spatial resolution, 1.29mm*1.29mm*2mm, BBTI, 1500ms (phantoms A and C) and 1800ms (phantom B). A previous normal volunteer study revealed background signal suppression including visceral fat and organs under these parameter settings for both MRAs.5 All phantom models were imaged under pulsatile glycerin water flow with amount of 67 to 450 ml/min according to a previous study.6
Image analysis.
In phantom A, signal degrading rate (SDR) of the distal segments to the proximal segments of the stenosis was evaluated for each branch. In phantom B, SDR of the outflow segments to the inflow segments of the aneurysm and coefficient of variation (CV) of signal intensity in the aneurysm was measured. In phantom C, SDR of the in-stent or distal segments to the proximal segments of the placed stent was evaluated.
Computer fluid dynamics (CFD) simulation.
In phantom B, CFD simulation was conducted to understand the flow patterns in the aneurysm. For boundary conditions, the same pulsatile flow setting as the image acquisition was given at the inlet and zero-pressure was given at the outlet.
Statistical analysis.
In phantom A, a Wilcoxon signed-rank test was used to compare variables between two image data sets. a p value < 0.05 was considered to be statistically significant.
Results
In phantom A, UTE MRA with minimal signal loss at the distal segment demonstrated significantly lower SDR than SSFP MRA (p<0.01). Configurations of the stenotic sites were maintained in UTE MRA. Signal decay at and distal to the stenosis appeared to be flow-direction dependent in SSFP MRA (Fig.3). In phantom B, both MRAs depicted outflow segments with minimal SDR. However, UTE MRA demonstrated homogeneous signal intensity in the aneurysm compared with SSFP MRA. UTE MRA provided lower CV in the aneurysm than SSFP MRA (0.27 for SSFP MRA and 0.07 for UTE MRA, respectively) (Fig.4). There was a turbulent flow pattern appeared in the aneurysm on the CFD model. In phantom C, SSFP MRA poorly visualized any in-stent or distal segments (Fig.5). SDRs in SSFP MRA were 83%-97% for in-stent segments and 65%-96% for distal segments, respectively. UTE visualized all segments expect for in-stent lumen in the stainless-steel stent. SDRs in UTE MRA were -1%-93% for in-stent segments and -4%-17% for distal segments, respectively.Discussion
In our phantom models, the following mechanism could be considered to cause intra-voxel spin dephasing: 1) accelerated flow at the stenosis, 2) turbulent flow in the aneurysm and 3) susceptibility effects from metallic devices. Such phenomenon might result in signal decay in SSFP MRA. On the contrary, UTE MRA provided minimal signal decay in all phantom models expect for in-stent segment of stainless-steel stent, indicating robustness of UTE MRA for the visceral arterial conditions associated with endovascular interventions. A potential limitation in this study included no evaluation of spatial resolution that might have influenced visibility of smaller vessels. However, lumen sizes in our phantom models were determined by clinical settings where endovascular interventions were performed. Therefore, we did not target smaller vessels in this study.Conclusion
UTE time-SLIP MRA demonstrated better visualization of phantom lumens simulating visceral artery diseases than SSFP time-SLIP MRA. Our results revealed a potential of UTE time-SLIP MRA for the evaluation of visceral artery diseases indicated for endovascular interventions.Acknowledgements
NoneReferences
1. Larson, R. A., Solomon, J. & Carpenter, J. P. Stent graft repair of visceral artery aneurysms. J. Vasc. Surg. 36, 1260–1263 (2002).
2. Sachdev, U. et al. Management of aneurysms involving branches of the celiac and superior mesenteric arteries: A comparison of surgical and endovascular therapy. J. Vasc. Surg. 44, 718–724 (2006).
3. Irie, R. et al. Assessing Blood Flow in an Intracranial Stent: A Feasibility Study of MR Angiography Using a Silent Scan after Stent-Assisted Coil Embolization for Anterior Circulation Aneurysms. American Journal of Neuroradiology 36, 967–970 (2015).
4. Takano, N. et al. Usefulness of Non–Contrast-Enhanced MR Angiography Using a Silent Scan for Follow-Up after Y-Configuration Stent-Assisted Coil Embolization for Basilar Tip Aneurysms. American Journal of Neuroradiology 38, 577–581 (2017).
5. Mori, R. et al. Ultrashort TE Time-Spatial Labeling Inversion Pulse MR Angiography with Deep Learning Reconstruction for Abdominal Visceral Arteries: A Feasibility Study. In: Proc 27th Annual Meeting ISMRM, Montreal (2018).
6. Sahbaee, P., Segars, W. P., Marin, D., Nelson, R. C. & Samei, E. The Effect of Contrast Material on Radiation Dose at CT: Part I. Incorporation of Contrast Material Dynamics in Anthropomorphic Phantoms. Radiology 283, 739–748 (2017).
Figures