1318
Ferumoxytol contrast increases the normalized relative difference in T1 reactivity between remote and ischemic myocardium
Caroline M. Colbert1,2, Anna Le3, Jiaxin Shao1, Jesse Currier3, Peng Hu1, and Kim-Lien Nguyen1,2,3
1Department of Radiological Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 2Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 3Division of Cardiology, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
1Department of Radiological Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 2Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 3Division of Cardiology, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
Synopsis
T1 reactivity can be used as a marker for myocardial perfusion reserve in the setting of ischemia or hypoperfusion. We hypothesize that ferumoxytol, as a pure intravascular agent with high r1 relaxivity, sensitizes T1 reactivity for assessment of myocardial perfusion. We selectively induced acute myocardial hypoperfusion in twelve healthy male Yorkshire swine. We then performed native and ferumoxytol-enhanced adenosine stress testing with the MOLLI sequence at 3.0T. Ferumoxytol increased absolute T1 reactivity in remote regions by 4.62-fold. The normalized difference in T1 was 4.5-fold greater in FE images compared to native T1.
Introduction
In ischemic heart disease (IHD), narrowing of the epicardial coronary arteries leads to myocardial hypoperfusion. Moderate to severe coronary narrowing can lead to a blunted myocardial perfusion reserve in the setting of exercise- or pharmacologic-induced stress. In cardiac magnetic resonance imaging (MRI), T1 reactivity [1] [2], which reflects the relative difference in tissue T1 between rest and vasodilator stress, can be used as a marker for reduced perfusion reserve in the setting of ischemia or hypoperfusion. Previous work shows that native T1 reactivity can be used effectively to distinguish between remote and ischemic myocardium [3]. As an ultrasmall superparamagnetic iron oxide ion, ferumoxytol can be used off-label as an MR contrast agent [4] to sensitize myocardial T1 to vasodilator-induced stress [5]. We hypothesize that in addition to sensitizing myocardial T1 to vasodilator stress, ferumoxytol increases the normalized relative difference in T1 reactivity between remote and ischemic myocardial regions, thereby increasing the conspicuity of ischemic defects. Our aim is to determine the relative difference in T1 reactivity of remote and ischemic myocardium using native and ferumoxytol-enhanced (FE) stress T1 mapping in swine models of acute myocardial hypoperfusion.Methods
We selectively induced acute myocardial hypoperfusion in twelve healthy male Yorkshire swine using either a partially inflated coronary angioplasty balloon in the LAD (n=3) or a 3D printed intracoronary stenosis implant in the LAD (n=8) or LCX (n=1). We then performed native and ferumoxytol-enhanced adenosine stress testing (Figure 1). A left ventricular short-axis stack of T1 maps (base, mid, apex) was acquired at rest and at peak pharmacologic stress (adenosine, 300 µg/kg/min, 4 min infusion) using the 5(3)3 MOLLI sequence in all swine (n=12) [FOV = 741 x 1522 mm, matrix size = 384 x 308, TR = 349 ms, TE = 1.08 ms, slice thickness = 8 mm, pixel bandwidth = 1085, flip angle = 35˚]. We used the Instantaneous Signal Loss simulation (InSiL) algorithm for T1 fitting of MOLLI images in order to minimize T1 error at high heart rates (>80 bpm) [6]. We contoured a single region of ischemic myocardium and a corresponding remote region in the contralateral myocardial segment in each subject. Statistical analyses were performed in MedCalc version 19.0.5 (MedCalc Software, Ostend, Belgium). The D’Agostino-Pearson test was used to assess normality of the data. T1 reactivity was compared using either the student’s t test or Wilcoxon signed-rank test according to normality.Results
No ferumoxytol-related adverse events occurred. At baseline, subjects showed an average heart rate of 88.3±12.5 bpm (beats per minute), systolic blood pressure of 101±10 mmHg and diastolic blood pressure of 61.4±9.1 mmHg. At peak adenosine infusion, subjects showed an average heart rate of 86.3±14.1 bpm, systolic blood pressure of 76±12 mmHg, and diastolic blood pressure of 40±7 mmHg. Ferumoxytol increased absolute T1 reactivity in remote regions by a factor of 4.62 (p<0.0001). FE T1 reactivity in ischemic regions remained blunted as expected. Both native and FE T1 reactivity were significantly different between ischemic and remote regions. Native T1 reactivity values in ischemic and remote regions were 1.6±1.0% vs 3.1±1.7% (p=0.0008), respectively. FE T1 reactivity values in ischemic and remote regions were -4.5±5.0% and ‑11.1±7.0% (p<0.0001), respectively. Relative to remote regions, absolute FE T1 reactivity was blunted in ischemic regions. The normalized difference in T1 reactivity, defined as (ReactivityRemote – ReactivityIschemic) / ReactivityIschemic, was 4.5-fold greater in FE images (FE: 401±589% vs native: 89±235%, p=0.0038.)Discussion
Myocardial T1-mapping is used to diagnose a range of cardiovascular pathologies. This study investigated the effect of ferumoxytol on T1 reactivity, which reflects the relative increase or decrease in T1 caused by vasodilator-induced stress. Adenosine induced vasodilation typically causes an increase in native myocardial T1, presumably due to expansion in the vascular compartment. Ferumoxytol shortens the T1 with adenosine-induced vasodilation. Our results demonstrate that native and FE T1 mapping can both distinguish between remote and ischemic myocardial regions in swine models of acute myocardial hypoperfusion. However, ferumoxytol increases the dynamic range of T1 reactivity as a measure of vasodilator response, causing a significant increase in absolute T1 reactivity in remote myocardium. As a result, ferumoxytol also increases the normalized difference in T1-reactivity between remote and ischemic myocardial regions. This increased contrast in T1-reactivity between remote and ischemic myocardium may enhance the visual conspicuity of ischemic defects, potentially making ferumoxytol-enhanced stress T1 mapping a more sensitive diagnostic tool.Conclusion
Ferumoxytol contrast increases the dynamic range of T1 reactivity as a measure of myocardial vasodilator response. Ferumoxytol also increases the normalized difference between remote and ischemic myocardium, increasing the visual conspicuity of ischemic defects.Acknowledgements
This work was supported by American Heart Association Transformational Award 18TPA34170049, and pilot funding from the UCLA Department of Medicine. We thank the UCLA Lux Lab for providing 3D printing services.References
- A. Liu et al., “Adenosine Stress and Rest T1 Mapping Can Differentiate between Ischemic, Infarcted, Remote, and Normal Myocardium Without the Need for Gadolinium Contrast Agents,” JACC Cardiovasc. Imaging, vol. 9, no. 1, pp. 27–36, 2016.
- S. K. Piechnik, S. Neubauer, and V. M. Ferreira, “State-of-the-art review: stress T1 mapping-technical considerations, pitfalls and emerging clinical applications.,” MAGMA, vol. 31, no. 1, pp. 131–141, Feb. 2018.
- M. Van Assen, R. Van Dijk, D. Kuijpers, R. Vliegenthart, and M. Oudkerk, “T1 reactivity as an imaging biomarker in myocardial tissue characterization discriminating normal , ischemic and infarcted myocardium,” Int. J. Cardiovasc. Imaging, no. 0123456789, 2019.
- G. B. Toth et al., “Current and potential imaging applications of ferumoxytol for magnetic resonance imaging.,” Kidney Int., vol. 92, no. 1, pp. 47–66, 2017.
- K.-L. Nguyen et al., “Ferumoxytol-Enhanced CMR for Vasodilator Stress Testing: A Feasibility Study,” JACC Cardiovasc. Imaging, p. 2950, Mar. 2019.
- J. Shao, K. L. Nguyen, Y. Natsuaki, B. Spottiswoode, and P. Hu, “Instantaneous signal loss simulation (InSiL): An improved algorithm for myocardial T1 mapping using the MOLLI sequence,” J. Magn. Reson. Imaging, vol. 41, no. 3, pp. 721–729, Mar. 2015.
Figures
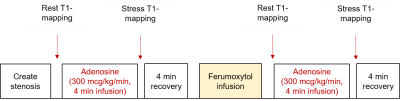
T1-mapping stress
testing protocol. Following localizers, we acquired a left ventricular short-axis stack of
T1 maps (base, mid, apex) at rest and at peak pharmacologic stress (adenosine,
300 µg/kg/min, 4 min infusion) before and after ferumoxytol infusion (4mg/kg
over 12-minutes).
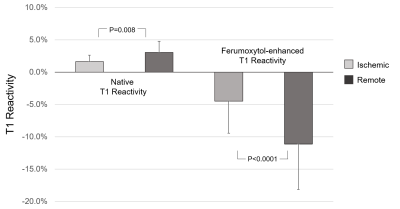
Focal T1 reactivity
in remote and ischemic myocardium of 12 swine. Remote and ischemic
regions demonstrate a significant difference in T1 reactivity on native and ferumoxytol-enhanced MOLLI images. P<0.05 significant. Mean + 1 SD.