1315
High-Resolution Free-Breathing Quantitative Myocardial Perfusion MRI Using Multi-Echo Dixon1MR R&D – Clinical Science, Philips Healthcare, Best, Netherlands, 2Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 3Philips Healthcare, Guildford, Surrey, United Kingdom, 4School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 5Philips Healthcare Iberia, Madrid, Spain, 6Philips Research Europe, Hamburg, Germany, 7Department of Medical and Health Sciences, Linkoping University, Linkoping, Sweden
Synopsis
First-pass perfusion cardiac MR (FP-CMR) allows the detection of myocardial ischemia. Also, quantitative methods enable a reliable and operator-independent assessment of myocardial perfusion. However, conventional FP-CMR has limited spatial resolution and should be performed under breath-hold. Therefore, diagnostic accuracy is compromised by respiratory induced motion artifacts and false-positive defects due to dark-rim artifacts. We propose, a k-t accelerated dual-saturation FP-CMR multi-echo Dixon sequence to increase the spatial resolution, estimate respiratory motion from fat images and measure T2*-related signal loss from the multi-echo images. Thus, perfusion quantification is improved by minimizing dark-rim artifacts, correcting for respiratory motion and T2*.
Introduction
First-pass perfusion cardiac MR (FP-CMR) is a non-invasive approach to detect coronary artery disease. However, FP-CMR requires breath-hold acquisitions to reduce respiratory motion and can suffer from low spatial resolution, which can lead to dark-rim artifacts that mimic hypoperfusion1,2. Furthermore, pixel-wise quantification analysis can be compromised by these artifacts as well as the non-linearity between the measured signal and contrast agent concentration1-4.Recently, a FP-CMR multi-echo Dixon (mDixon) sequence was proposed to address respiratory motion and T2*-related signal loss5. From the fat-only images, unaffected by the contrast bolus, respiratory motion is estimated and the diagnostic water images are corrected using image registration. Moreover, the multi-echo images are used for T2* correction of the arterial input function (AIF) to further improve perfusion quantification. However, this approach was limited to low-resolution images, which can lead to dark-rim artifacts.
In this work, we propose a k-t accelerated dual-saturation6 single-bolus FP-CMR mDixon approach, which combines dynamically variable Cartesian undersampling with a motion-corrected reconstruction with low-rank and sparse constraints7, to achieve high-resolution FP-CMR images. Moreover, a dual-saturation single-bolus acquisition strategy is used to acquire a low-resolution image with low T1-sensitivity for the AIF, followed by a high-resolution myocardial image. The proposed fat-water separation for motion-corrected spatio-temporally accelerated myocardial perfusion (FOSTERS) approach was tested in 4 patients with suspected cardiovascular disease.
Methods
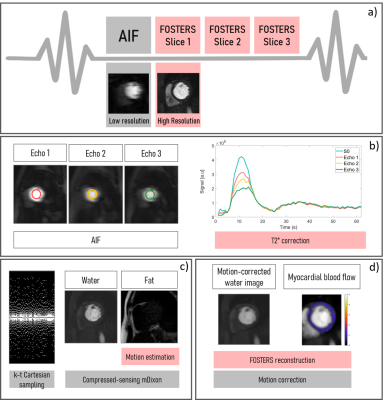
Acquisition:The schematic of the proposed FOSTERS framework is shown in Figure 1. Three patients were scanned during rest with 6-fold accelerated FOSTERS during the first-pass of bolus injection (0.075 mmol/Kg of Gadobutrol at 4ml/s followed by 25ml saline flush) on a 3T Philips Achieva scanner. A free-breathing ECG-triggered dual-saturation single-bolus turbo field echo with three echoes per excitation pulse sequence was used to acquire three short-axis slices (basal, mid and apical). The following parameters were used for patients 1-3: FOV = 320 x 300 mm2, in-plane resolution = 1.6 x 1.6 mm2, slice thickness = 10 mm, TR = 4.2 ms, TE1/TE2/TE3 = 1.3/2.3/3.2 ms, saturation delay = 100 ms, flip angle = 15°, shot length = 130.2 ms. For comparison, Patient 1 was also scanned with a routine low-resolution FP-CMR acquisition6 with the following imaging parameters: spatial resolution = 2.6 × 2.6 × 10 mm3, FOV = 360 x 360 mm2, TR / TE = 2.1 / 1.08 ms, CS-SENSE = 3.2. Patient 4 was scanned using an 8-fold accelerated two-echo FOSTERS acquisition with FOV = 360 x 320 mm2 and TR/TE1/TE2 = 3.2/1.3/2.2 ms, shot length = 80.0 ms. The total acquisition time for all the acquisitions was 60 seconds.
Reconstruction and Analysis:
The FOSTERS reconstruction framework was implemented inline using the scanner software. The main steps are: 1) mDixon compressed sensing (CS) reconstruction to generate water and fat images; 2) The fat-only images are used to estimate the frame-by-frame respiratory motion by registering every frame to the mean over all frames8; 3) Translational motion correction is performed directly in k-space by applying a linear phase shift to the multi-echo images; 4) Motion-corrected diagnostic water images are generated using a reconstruction with low-rank and sparsity constraints7.
For each echo, the AIF was found using a region of interest drawn in the left ventricle and T2* was estimated by fitting the mean intensity for each echo to $$$S(t) = S_{0}\mathrm{e}^{-TE/T2^{*}}$$$, where $$$S_0$$$ is the signal without T2* decay. Finally, FP-CMR images were automatically segmented using a deep learning-based method and myocardial blood flow (MBF) maps were generated using a Bayesian inference method9.
Results
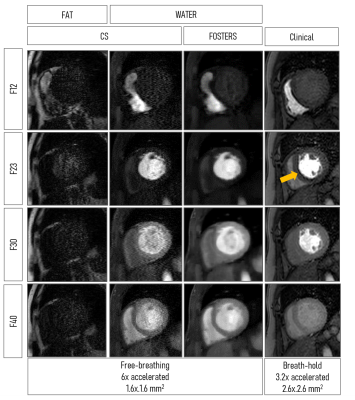
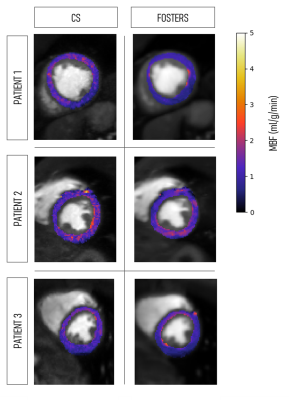
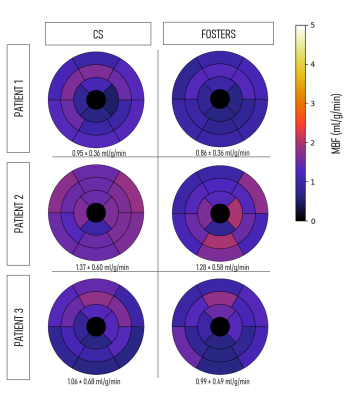
A comparison between fat- and water-only images obtained with CS and FOSTERS, as well as the clinical FP-CMR images, are shown in Figure 2 for one patient (Patient 1). The FOSTERS framework minimizes the noise and produces high-quality FP-CMR images. Improved myocardial delineation across all the dynamic images was observed in all patients with FOSTERS.Figure 3 shows MBF maps for three patients using CS and FOSTERS. CS images exhibit higher levels of noise resulting in less homogenous MBF maps. For Patient 2, the high values of MBF can be explained by the presence of residual in-plane motion. The 16-segment bullseye plots generated from CS and FOSTERS images are shown in Figure 4. The average MBF is within the expected range for rest perfusion scans for subjects without perfusion defects. Furthermore, the variability of MBF values over the segments was generally reduced with FOSTERS.
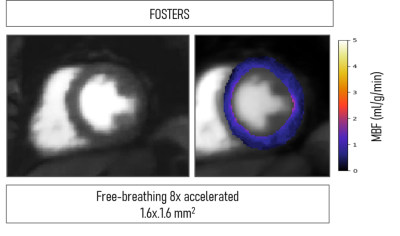
Figure 5 shows the high-resolution image generated with FOSTERS and the respective MBF map. For this patient, FOSTERS acquisitions were performed using an 8-fold acceleration and two-echo acquisition due to the high heart rate. Despite that, high-quality images and MBF maps could still be obtained. This preliminary result shows that FOSTERS can be accelerated further to allow higher spatial resolution, higher heart rate and/or shorter acquisition windows to minimize cardiac motion.
Conclusion
High-resolution free-breathing quantitative myocardial perfusion MRI is enabled by combining a dual-saturation single-bolus FP-CMR mDixon sequence with k-t Cartesian undersampling, motion-correction, and reconstruction with low-rank and sparsity constraints. The proposed framework generates high-quality diagnostic water images with minimal dark rim artifact. Furthermore, the multi-echo images are used for T2* correction and hence, to improve myocardial blood flow quantification.Acknowledgements
This work was supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z], by the European Commission within the Horizon 2020 Framework through the MSCA-ITN-ETN European Training Networks (project number 642458), and by the Swedish Research Council (2018-04164).References
- Kellman P, Arai AE. Imaging sequences for first pass perfusion - a review. J Cardiovasc Magn Reson. 2007;9(3):525-37.
- Jerosh-Herold M. Quantification of myocardial perfusion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12(57):12-57.
- Benovoy M, Jabocs M, Cheriet F, et al, Robust universal nonrigid motion correction framework for first-pass cardiac MR perfusion imaging, JMRI. 2017; 46(4):1060-1072.
- Kellman P, Hansen MS, Nielles-Vallespin S, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19(1):43.
- Henningsson M, Farias A, Villa A, et al. Quantitative myocardial perfusion using multi-echo Dixon for respiratory motion correction and arterial input function estimation. ISMRM 2018. 0764.
- Sanchez-Gonzalez J, Fernandez-Jimenez R, Nothnagel N, et al. Optimization of dual-saturation single bolus acquisition for quantitative cardiac perfusion and myocardial flow maps. J Cardiovasc Magn Reson. 2015;17:21.
- Otazo R, Kim D, Axel L, Sodickson D. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010;64(3):767-776.
- Kabus S, Lorenz C. Fast elastic image registration. MICCAI 2010. 81-89.
- Scannell C, Chiribiri A, Villa A, et al. Hierarchical Bayesian myocardial perfusion quantification. 2019; arXiv:1906.02540
Figures