1294
Hybrid structure-function connectome predicts individual cognitive abilities1Radiology, Weill Cornell Medicine, New York, NY, United States, 2Neuroscience, Weill Cornell Medicine, New York, NY, United States, 3Sarah Lawrence College, Bronxville, NY, United States, 4Biological and Biomedical Engineering, McGill University, Montreal, QC, Canada, 5Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal, QC, Canada, 6Psychiatry, McGill University, Montreal, QC, Canada
Synopsis
Structural connectivity (SC) and functional connectivity (FC) can be independently used to predict cognition and show distinct patterns of variance in relation to cognition. No work identified has yet investigated whether SC and FC can be combined to better predict cognitive abilities. In this work, we aimed to predict cognitive measures in 785 healthy adults using a hybrid structure-function connectome and quantify the most important connections. We show that: 1) hybrid connectomes explain 15% of the variance in individual cognitive measures, and 2) long-range cortico-cortical functional connections and short-range cortico-subcortical and subcortico-subcortical structural connections are most important for the prediction.
Introduction
Functional connectivity (FC) represents temporal dependency patterns between regional blood-oxygenation-level dependent (BOLD) activity in functional magnetic resonance imaging (fMRI) time series, and structural connectivity (SC) represents the inter-regional white matter pathways estimated from diffusion-weighted MRI (dwMRI). FC [1-3] and SC [4-7] have both been linked to cognitive functioning, and independently used to predict cognitive measures [8-11]. FC reflects performance variability in several cognitive domains such as executive control [8], and intellectual performance [9], and can be used to successfully predict a range of individual cognitive measures [10]. Moreover, it has been shown that measures extracted from structural and diffusion images can explain variability in intelligence quotient [12], and SC can be used to make accurate cognitive predictions [11]. Thus, SC and FC may hold promise to reveal structural and functional correlates of cognitive abilities. While it has been shown that SC and FC show unique and distinct patterns of variance in relation to cognition across subjects [11], no work identified has yet investigated whether SC and FC information can be combined to better predict cognitive measures. The purpose of this study was to: 1) Predict cognition in 785 healthy adults using a hybrid structure-function connectome, and 2) Quantify the most important pairwise structural and functional connections for cognitive prediction.Methods
Using publicly available data from the Human Connectome Project, we examined resting-state BOLD fMRI time series data and dwMRI from 785 healthy young adults (ages 22-37). Zero-lag Pearson correlation with regression of the global signal and its temporal derivative were used to generate a resting-state FC matrix for each subject using an 86 region FreeSurfer atlas (34 cortical and 9 subcortical areas per hemisphere). Each subject’s FC matrix was Fisher’s z-score transformed. Streamline counts between region-pairs based on probabilistic tractography were used to generate a SC matrix for each subject. Each subject’s SC was then resampled to a Gaussian distribution [13] as follows: given N raw data values x1, …, xN, N random samples r1, …, rN, from a Gaussian distribution with a mean of 0.5 and a standard deviation of 0.1 were generated. The smallest raw data value was replaced with the smallest randomly sampled value, and this was repeated until all raw data values were replaced. This produced a set of N resampled data values with a Gaussian distribution. Cognitive measures used in this study were the following composite scores: crystallised, early childhood, fluid, and total, all of which are based on an individual’s performance on different NIH Toolbox tests. Machine learning prediction of cognitive measures was completed using three different input data sets: 1) FC, 2) SC, and 3) hybrid structure-function connectome [14]. When using only FC or SC, the upper triangular portion of the matrices were vectorised and used as the model input. For the hybrid structure-function connectome, the vectorised upper triangular portion of the SC and the vectorised lower triangular portion of the FC were concatenated. The data were separated into training (80%) and testing (20%) subsets and the training set was used to train an elastic net regression model. For each cognitive measure, hyperparameter tuning of the L1 penalty ratio and the regularisation parameter was conducted using nested grid search cross validation with 5-fold inner and outer folds. The optimised hyperparameters were identified and used to fit a single final model. This model was trained used the entire training subset and the feature importance was extracted. This model was then evaluated on the test subset and the explained variance score was calculated. This was repeated using the same 10 permutations of randomised training and testing splits for all four cognitive measures to get a distribution of overall performance metrics. The feature weights obtained for each cognitive measure were averaged across the permutations to get a mean feature importance value.Results
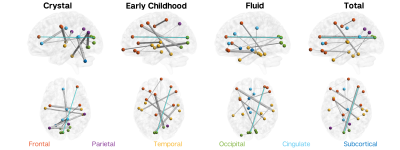
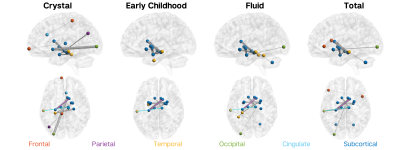
FC alone is able to explain 11.3%, 6.5%, 7.5%, and 12.0% of the variance in crystallised, early childhood, fluid, and total cognition composites, respectively. SC alone is able to explain 5.3%, 3.0%, 2.3%, and 7.1% of the variance in crystallised, early childhood, fluid, and total cognition composites, respectively. The hybrid structure-function connectome is able to explain 12.5%, 8.2%, 8.3%, and 15.0% of the variance in crystallised, early childhood, fluid, and total cognition composites, respectively. The most important FC features for cognitive prediction are primarily long-range inter-hemispheric cortico-cortical connections (Figure 1), while the most important SC features are primarily short-range inter-hemispheric cortico-subcortical and subcortical-subcortical connections (Figure 2). Pearson’s correlation between the feature importance for FC and SC features are 0.015, -0.016, -0.021, and -0.017 for crystallised, early childhood, fluid, and total cognition composites, respectively.Discussion
The hybrid structure-function connectome outperforms the independent use of SC for cognitive predictions. This demonstrates that FC-based predictions of cognitive measures are complementary to SC-based predictions. Moreover, there was no correlation between the feature importances of the pairwise SC and FC features. This indicates that while a given region-pair’s FC might be important for the prediction of cognitive measures, the same region-pair’s SC may not be important. Taken together, this suggests that the integration of multi-modal data is crucial to understanding the neurophysiological correlates of cognitive function.Acknowledgements
Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
This work was supported by the following grants: R21 NS104634-01 (AK) and R01 NS102646-01A1 (AK).
References
1. Song, M., et al., Default network and intelligence difference. Conf Proc IEEE Eng Med Biol Soc, 2009. 2009: p. 2212-5.
2. Song, M., et al., Brain spontaneous functional connectivity and intelligence. Neuroimage, 2008. 41(3): p. 1168-76.
3. Pamplona, G.S., et al., Analyzing the association between functional connectivity of the brain and intellectual performance. Front Hum Neurosci, 2015. 9: p. 61.
4. Moeller, K., K. Willmes, and E. Klein, A review on functional and structural brain connectivity in numerical cognition. Front Hum Neurosci, 2015. 9: p. 227.
5. Willmes, K., K. Moeller, and E. Klein, Where numbers meet words: a common ventral network for semantic classification. Scand J Psychol, 2014. 55(3): p. 202-11.
6. Matejko, A.A., et al., Individual differences in left parietal white matter predict math scores on the Preliminary Scholastic Aptitude Test. Neuroimage, 2013. 66: p. 604-10.
7. Klein, E., et al., Considering structural connectivity in the triple code model of numerical cognition: differential connectivity for magnitude processing and arithmetic facts. Brain Struct Funct, 2016. 221(2): p. 979-95.
8. Seeley, W.W., et al., Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 2007. 27(9): p. 2349-56.
9. van den Heuvel, M.P., et al., Efficiency of functional brain networks and intellectual performance. J Neurosci, 2009. 29(23): p. 7619-24.
10. Li, J., et al., Global signal regression strengthens association between resting-state functional connectivity and behavior. Neuroimage, 2019. 196: p. 126-141.
11. Zimmermann, J., J.D. Griffiths, and A.R. McIntosh, Unique Mapping of Structural and Functional Connectivity on Cognition. J Neurosci, 2018. 38(45): p. 9658-9667.
12. Seidlitz, J., et al., Morphometric Similarity Networks Detect Microscale Cortical Organization and Predict Inter-Individual Cognitive Variation. Neuron, 2018. 97(1): p. 231-247 e7.
13. Honey, C.J., et al., Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A, 2009. 106(6): p. 2035-40.
14. Amico, E. and J. Goñi, Mapping hybrid functional-structural connectivity traits in the human connectome. Network Neuroscience, 2018. 2(3): p. 306-322.
Figures