1277
Considerations for Cardiac Phase-Specific B0 Shimming at 7 T1Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital Wuerzburg, Wuerzburg, Germany, 2Department of Internal Medicine I, University Hospital Wuerzburg, Wuerzburg, Germany, 3Departments of Biomedical Engineering and Radiology, Columbia University, New York City, NY, United States
Synopsis
Spatio-temporal inhomogeneities of the static magnetic (B0) field are a major limiting factor in cardiac magnetic resonance applications at 7T. A previously developed shim strategy was demonstrated to correct spatial myocardial B0-field inhomogeneities in a preliminary in vivo implementation. To correct localized spots of B0-inhomogeneities, third-order terms were found to be beneficial. Cardiac phase-specific shimming was evaluated in simulations based on the in vivo field map data, and optimal shim settings were shown to differ between cardiac phases. Future work will address the application of a shim averaged over all cardiac phases to each individual phase.
Introduction
Susceptibility differences of the myocardial tissue and surrounding lungs induce inhomogeneities of the static magnetic (B0) field, which lead to signal loss and image distortions in human cardiac magnetic resonance (CMR) at 7T1. These B0-inhomogeneities also vary temporally over different cardiac phases2 and breath-hold positions3. However, a homogeneous B0-field within the heart is required for a number of applications including T2*-mapping to analyze microstructure4, steady-state free precision CINE imaging to assess cardiac function5, or fast imaging techniques such as spiral readout trajectories6 and echo-planar imaging7. Very importantly at 7T, the peak amplitudes of radiofrequency pulses are optimized for a target range of B0-field variations8. To correct, i.e. shim the cardiac B0-inhomogeneities, a dedicated strategy was recently developed9. This strategy consists of the triggered field map acquisition, the anatomy-matched shim-region-of-interest (SROI) selection, and the calibration-based spherical harmonics (SH) B0-field modeling in dedicated software10. Pilot results from the in vivo implementation demonstrated the capability to shim large myocardial B0-inhomogeneities11. In this work, we further evaluate the role of third-order shim terms for CMR at 7T as well as the potential benefit of extending this static B0-shim to cardiac phase-specific shimming (CPSS)12,13, since higher-order dynamic CPSS is not a standard vendor-supplied tool.Methods
All field map data was acquired with a 1TX/16RX thorax coil (MRI Tools) on a 7T whole-body MR system (MAGNETOMTM Terra, Siemens Healthineers). The scanner is equipped with full second- and partial third-order SH shims Z3, Z2X, Z2Y and Z(X2-Y2). Cardiac triggering was based on acoustic (ACT, MRI Tools) and ECG monitoring of the heartbeat. Three mid-ventricular transversal slices were acquired for six healthy volunteers (average age 24 years, average weight 75 kg) after approval by the local ethics committee (7/17-sc). Parameters for the multi-echo GRE pulse sequence were TE=1.69/2.83/3.96/6.07/9.72 ms, matrix size 128×128, FOV=300×300 mm2, TR=400 ms, slice thickness=6 mm. B0-maps were reconstructed voxel-wise by the phase method in B0DETOX software10, which was extended by spatial phase-unwrapping (FSL library)14. Anatomy-matched SROI selections and calibration-based B0-field modeling were carried out within the shim software. Further calculation of standard deviations (SD) and interquartile ranges (IQR) of the B0 distributions was performed in MATLAB R2015b (MathWorks Inc, USA).Results
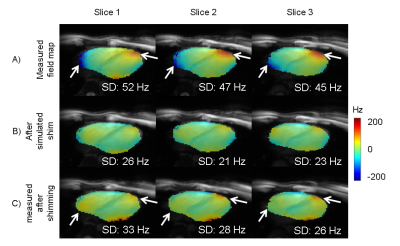
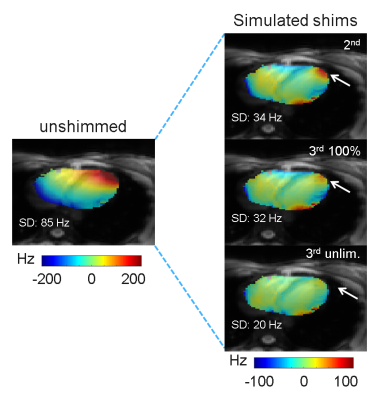
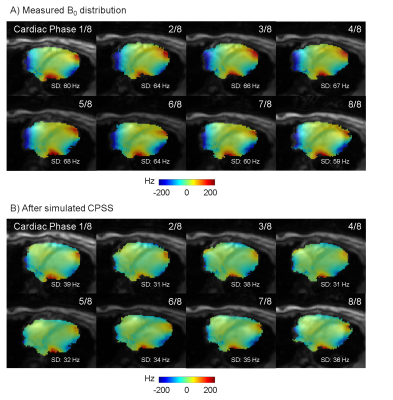
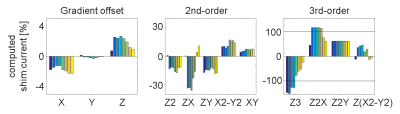
The B0-field within the complete left ventricle (LV) showed a largely homogeneous pattern (Fig. 1). However, extreme spatial B0-inhomogeneities were found within the myocardium such as the anterolateral region (Fig.1C,D). Localized spots of B0-inhomogeneities showed a temporal variation, which is visible from the same slice acquired at a different cardiac phase (Fig. 1E,F). For the left-ventricular myocardium, this variation accounted for a ΔSD of 10±5 Hz and ΔIQR of 19±12 Hz between systolic and diastolic phases (mean ± standard deviation) for the six subjects. First in vivo shimming in a healthy subject demonstrated the capability of the developed shim strategy to correct large myocardial B0-inhomogeneities at a delay of 400 ms post R wave (Fig. 2). Simulation results (Fig. 2B) matched well with the residual B0-field remaining after the experimental third-order shim (Fig. 2C). B0-field homogeneity was improved within the anterolateral segment of the LV and the lateral segment of the right ventricle (Figure 2, arrows). Smaller localized B0-inhomogeneities remained within the myocardium. The role of higher-order SH terms was evaluated for the scanner-integrated third-order terms (Fig. 3). A comparison between second-order only and second- plus third-order shims demonstrated that, though the reduction of SD for the overall heart was small, the third-order terms were in particular beneficial to correct the myocardial B0-inhomogeneities in the anterolateral segment of the LV. However, residuals of the third-order terms remained due to limited hardware dynamic ranges of 0.68 Hz/cm3 for Z3, 0.35 Hz/cm3 for Z2X, 0.34 Hz/cm3 for Z2Y, and 0.10 Hz/cm3 for Z(X2-Y2). The second-order terms did not exceed the hardware limitations for any shim. To address the temporal B0-field variations, a shim was computed for eight cardiac phases (Fig. 4). B0-field homogeneity was improved by the simulated CPSS by 29±6 Hz for all phases. The corresponding currents varied from phase to phase (Fig. 5). Z, which had a dynamic range of 2129 Hz/cm, was on average employed on 1.82±0.77% with a peak-to-peak range of 1.91%. The third-order terms were used an average on -96±48% for Z3, 96±30% for Z2X, and 18±25% for Z(X2-Y2).The corresponding peak-to-peak ranges were 127%, 72%, and 58%, respectively.Discussion
Extreme spatial B0-inhomogeneities within the heart were located in localized spots within the myocardium, and showed a temporal variation over the cardiac cycle. A calibration-based third-order shim strategy for an anatomy-matched SROI was developed and initial in vivo implementation demonstrated its feasibilityfor shimming at individual cardiac phases. The scanner-integrated third-order terms were advantageous to obtain a homogeneous B0-field. Simulations of CPSS for one volunteer resulted in not negligible shim current variations. Analysis of other subjects will allow a more accurate determination of the required shim amplifiers, which are not integrated in the MR system up-to-date. Future work will address the application of a shim averaged over all cardiac phases to each individual phase.Conclusion
Detrimental B0-inhomogeneities at myocardial MRI at 7T can be reduced with a dedicated third-order, ECG-gated shim strategy using calibration-based SH B0-field modeling. Computed cardiac phase-specific shims differed over the cardiac cycle.Acknowledgements
Financial support was obtained from the German Ministry of Education and Research (BMBF) under grant #01EO1504.References
[1] Snyder CJ, DelaBarre L, Metzger GJ, Van de Moortele PF, Akgun C, Ugurbil K, Vaughan JT. Initial results of cardiac imaging at 7 Tesla. Magn Reson Med 2009;61:517-524.
[2] Jaffer FA, Wen H, Balaban RS, Wolff SD. A method to improve the B0 homogeneity of the heart in vivo. Magn Reson Med 1996;36:375-383.
[3] Schmitter S, Wu X, Ugurbil K, Van de Moortele PF. Design of Parallel Transmission Radiofrequency Pulses Robust Against Respiration in Cardiac MRI at 7 Tesla. Magn Reson Med 2015;74:1291-1305.
[4] Huelnhagen T, Hezel F, Serradas Duarte T, Pohlmann A, Oezerdem C, Flemming B, Seeliger E, Prothmann M, Schulz-Menger J, Niendorf T. Myocardial effective transverse relaxation time T2* correlates with left ventricular wall thickness: A 7.0 T MRI study. Magn Reson Med 2017;77:2381 – 2389.
[5] Schär M, Kozerke S, Fischer SE, Boesiger P. Cardiac SSFP imaging at 3 Tesla. Magn Reson Med 2004;51:799-806.
[6] Nayak KS, Cunningham CH, Santos JM, Pauly JM. Real-Time Cardiac MRI at 3 Tesla. Magn Reson Med 2004;51:655-660.
[7] Ding S, Wolff SD, Epstein FH. Improved Coverage in Dynamic Contrast-Enhanced Cardiac MRI Using Interleaved Gradient-Echo EPI. Magn Reson Med 1998;39:514-519.
[8] Tao Y, Hess AT, Keith GA, Rodgers CT, Liu A, Francis JM, Neubauer S, Robson MD. Optimized saturation pulse train for human first-pass myocardial perfusion imaging at 7T. Magn Reson Med 2015;73:1450-1456.
[9] Hock M, Stefanescu MR, Terekhov M, Lohr D, Herz S, Juchem C, Schreiber LM. Third-Order Cardiac B0-Shimming at 7 T in Humans. ISMRM Workshop on Ultrahigh Field Magnetic Resonance: Technological Advances, Translational Research Promises & Clinical Applications, Dubrovnik, 2019:3.
[10] Juchem C. B0DETOX – B0 Detoxification Software for Magnetic Field Shimming. Innovation.columbia.edu/technologies/cu17326_b0detox (2017). Columbia TechVenture (CTV), license CU17326.
[11] Hock M, Terekhov M, Reiter T, Lohr D, Juchem C, Schreiber LM. Correction of myocardial B0-inhomogeneities at 7 T with ECG-gated spherical harmonics shimming. 2019 Minnesota Workshop on High and Ultra-high Field Imaging, Minnesota, MN, USA, 2019.
[12] Kubach MR, Bornstedt A, Hombach V, Merkle N, Schär M, Spiess J, Nienhaus GU, Rasche V. Cardiac phase-specific shimming (CPSS) for SSFP MR cine imaging at 3 T. Phys Med Biol 2009;54:N467-N478.
[13] Mattar W, Juchem C, Terekhov M, Schreiber LM. Multi-coil B0 shimming of the human heart: a theoretical assessment. In: Proceedings of the 24th Annual Meeting of ISMRM, Singapore, 2016:1151.
[14] Smith SM, Jenkinson M, Woolrich MW, Beckman CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23:S208-S219.
Figures