1260
A novel interchangeable, double-tuned, twin head coil array design for 1H/23Na MR imaging and 1H/31P MR spectroscopy at 7 T1INM-4, Forschungszentrum Juelich, Juelich, Germany, 2INM-11, Forschungszentrum Juelich, Juelich, Germany, 3JARA-BRAIN-Translational Medicine, Aachen, Germany, 4Department of Neurology, RWTH Aachen University, Aachen, Germany
Synopsis
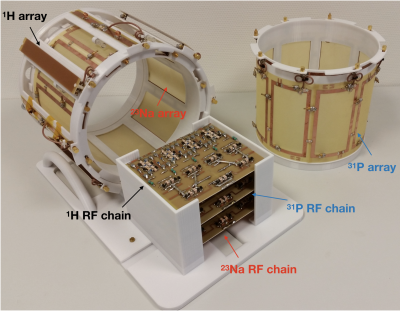
X-nuclei MR offers unique access to important metabolic information in tissues. Multi-tuned coils are required for the X-nuclei measurements, but designing a well-performing coil is challenging. In this study, we present our novel design and performance evaluation of an interchangeable, twin, double-tuned, head coil array for 1H/23Na MR imaging and 1H/31P MR spectroscopy at 7 T. The outer proton array was built using an alternatingly positioned 4-channel dipole antenna and a 4-channel microstrip transmission line array to improve decoupling. The inner 8-channel X-nuclei loop arrays, orthogonal to the 1H, were designed identically to enable conventionally switching between 23Na and 31P.
Introduction
Investigating X-nuclei (non-proton nuclei), e.g. sodium-23 (23Na) and phosphorus-31 (31P) has been of great interest recently with increasing the availability of ultra-high field systems. X-nuclei offer unique access to complementary and important biochemical and metabolic information in tissues, and alterations in these nuclei are strongly related to a variety of pathological and neurodegenerative conditions.1,2 However, studies using X-nuclei suffer from their intrinsically lower natural abundance and MR sensitivity compared to those of the protons, making any improvement in signal-to-noise ratio (SNR) desirable. B0 shimming with X-nuclei is also problematic; therefore, the concurrent acquisition of proton imaging is beneficial. The nuclear Overhauser effect (NOE) can also be used to boost SNR of, for example, 31P metabolites, and to increase spectral fitting accuracy.3 Multi-resonant RF coils are, therefore, likely to be used to attain the requirements, but designing a well-performing multi-tuned coil is challenging and a number of design approaches have been introduced previously.4-6 In this work, we present our novel design and performance evaluation of an interchangeable, twin, double-tuned, head coil for 1H/23Na MR imaging and 1H/31P MR spectroscopy at 7 T.Material and methods
As shown in Figure 1, our transmit/receive coil assembly consisted of a proton antenna array and an X-nuclei coil array which included either an 8-channel 23Na or a 31P loop array. The proton array was located in an outer shell (diameter/length = 300/220 mm) and the X-nuclei coil array (diameter/length = 260/170 mm) was placed inside the proton array. The proton array included 4-channel dipole antennas and 4-channel microstrip transmission lines (MTLs) - which were selected as they offer benefits in B1 penetration and symmetric excitation at ultra-high field.7,8 The dipole and MTLs were placed alternatively one next to another in order to improve decoupling.9 Both the 23Na and 31P array were individually tuned, but the design was identical, and both X-nuclei coil arrays could share the outer 1H array, and were exchangeable. In order to avoid any loss, the dipole or MTL element was located across the middle of each X-nuclei loop. This concept was chosen since the loop and either the dipole or the MTL are penetrated perpendicularly as they are geometrically isolated relating to 1H elements without requirement10 of lossy decoupling components, e.g. traps. The eight X-nuclei loops were capacitively decoupled between next neighbours.11In order to evaluate the performance of the proposed coil/antenna array, we first measured the S-parameters of each nucleus, and then carried out MR imaging and spectroscopy on a 7 T Siemens Terra. In the MR experiments, standard MR sequences were used, and the parameters were for 1H: 2D FLASH, TR = 8.6 ms, TE = 3.69 ms, Averages = 2, resolution = 0.5 × 0.5 × 5 mm3, acquisition time = 18.6 seconds, for 23Na: 3D FLASH, TR = 25 ms, TE = 3.63 ms, 32 averages, resolution = 4 × 4 × 4 mm3, acquisition time = 10:29 minutes and for 31P: 3D CSI, TR = 2000 ms, TE = 0.35 ms, 4 averages, weighted phase encoding scheme, voxel-of-interest (VOI) = 25 × 25 × 25 mm3, with and without applying NOE, acquisition time = 6 minutes. A 100 mM NaCl and 100 mM KH2PO4 doped water phantom was used for 23Na and 31P measurements. As an initial step, all proton and X-nuclei arrays channels were tested individually.
Results
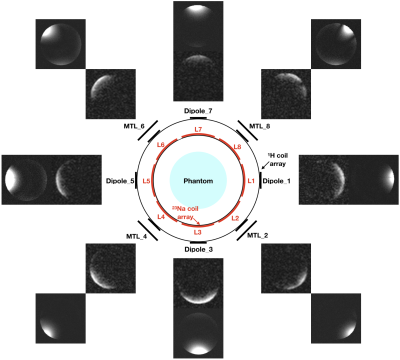
Figure 2 displays an S-parameter matrix of 1H, 23Na and 31P arrays measured on the bench using a vector network analyser. It can be seen that the decoupling, particularly in the proton channel, improved significantly (better than -20 dB) between adjacent elements. Without the proposed decoupling schemes or circuits, the value was, as expected, quite poor (around -7 dB).Figures 3 and 4 show the proton/sodium images and the 8 × 8 phosphorus spectra (without NOE) overlaid on the associated proton image acquired at all individual channels. This confirmed that all 24 channels were operating equitably. The SNR increase at the selected VOIs as a result of NOE enhancement is also shown in Figure 4.
Discussion
This study has successfully demonstrated the novelty and benefits of using the multi-tuned coil array design, and shown its feasibility for use in 1H/23Na MRI and 1H/31P MRS. The inner, twin X-nuclei arrays can be flexibly switched between 23Na and 31P, and potentially to any other nuclei of interest, e.g. 7Li, 13C or 17O. We found a negligible tuning variation of the proton elements when changing from the 23Na to the 31P array. The inner, 8-channel loop arrays can also be extended to 16-channels12, or more, in the z-axis to improve SNR or to shorten the scan time. The proton elements are independent and are well decoupled from each other, potentially improving the parallel transmit performance. To further investigate this, several tasks would also be required, for example, parallel imaging examination, additional evaluation relating to NOE enhancement3 and implementation of a whitened singular value decomposition13 spectra combination method. These will be studied in the future.Acknowledgements
No acknowledgement found.References
1. Shah NJ, et al. Imaging of sodium in the brain: a brief review. NMR Biomed. 2016;29(2):162-174.
2. Bonvento G, et al. Imaging and spectroscopic approaches to probe brain energy metabolism dysregulation in neurogenerative diseases. J Cerebr Blood F Met. 2017;37(6):1927-1943.
3. Peeters TH, et al. 3D 31P MR spectroscopic imaging of the human brain at 3T with a 31P receive array: an assessment of 1H decoupling, T1 relaxation times, 1H-31P nuclear Overhauser effects and NAD+. NMR Biomed. 2019.
4. van de Bank B, et al. Optimized 31P MRS in the human brain at 7T with a dedicated RF coil setup. NMR Biomed. 2015;28(11):1570-1578.
5. Brown R, et al. A nested phosphorus and proton coil array for brain magnetic resonance imaging and spectroscopy. Neuroimage. 2016;124:602-611.
6. Malzacher M, et al. Feasibility study of a double resonant 8-channel 1H/8-channel 23Na receive-only head coil at 3 tesla. Magn. Reson. Imaging. 2019;59:97-104.
7. Raaimakers AJ, et al. Dipole antennas for untrahigh-field body imaging: a comparison with loop coils. NMR Biomed. 2016;29(9):1122-1130.
8. Wu B, et al. Shielded microstrip array for 7T human MR imaging. IEEE Trans Med Imaging. 2010;29(1):179-184.
9. Yan X, et al. A mixed dipole and microstrip transmit/receive array. Proc. Int. Soc. Magn. Reson. Med. 2016, pp.2140.
10. Hong S-M, et al. Design of a quadrature 1H/31P coil using bent dipole antenna and four-channel loop at 3T MRI. IEEE Trans Med Imaging. 2018;37(12):2613-2618.
11. von Morze C, et al. An eight-channel, nonoverlapping phased array coil with capacitive decoupling for parallel MRI at 3T. Concepts Magn Reson Part B. 2007;31B(1):37-43.
12. Steensma BR, et al. An 8-channel Tx/Rx dipole array combined with 16 Rx loops for high-resolution functional cardiac imaging at 7 T. MAGMA. 2018;31(1):7-18.
13. Rodgers CT, Robson MD. Receive array magnetic resonance spectroscopy: whitened singular value decomposition (WSVD) gives optimal Bayesian solution. Magn. Reson. Med. 2010;63:881-891.
Figures