1259
Wearable MRS system for blood glucose measurement: Proof of concept1Radiology, New York University, New York, NY, United States, 2Endocrinology, New York University, New York, NY, United States
Synopsis
This study presents preliminary results supporting a new concept to build a wearable magnetic resonance spectroscopy (MRS) system for noninvasive blood glucose measurement in humans. Computer simulations, hardware buildings and subject studies demonstrated the promising of such a system.
INTRODUCTION
High blood glucose level is a characteristic of diabetes: 100-126 mg/dL for pre-diabetes and >126 mg/dL for diabetes1. Diabetes is a metabolic disease that causes acute and serious chronic complications1. It becomes a huge healthcare burden worldwide. In the United States there were 23.1 million people with diabetes in 2015 and $105 billion in annual direct cost 2. Globally, there were 422 million diabetics and $827 billion annual direct cost 3-6. There is no known cure for the disease1. Current management is to keep blood glucose level within acceptable range as close to normal (~100 mg/dL). This is usually achieved with healthy diet, exercise, weight loss, and medication. Monitoring glucose level therefore becomes a critical part of the management. Currently, daily monitoring is performed at home using a meter that requires finger-pricking for a drop of blood sample7. The procedure is invasive and can be painful, making patients less likely adhere to it. Minimally-invasive and less painful solutions have been developed in recent years, such as continuous glucose monitoring (CGM) meters8,9 which insert a small biosensor under the skin and measure glucose level in cellular fluid, not in blood, and thus need extra calibration by finger-pricking meter10. Noninvasive and painless solutions are under extensive development, including optical sensor11,12, smart contact lens13, radio-wave sensor14, and ultrasonic sensor15. These hopefuls however share a major technical drawback of non-specific to blood glucose and unlikely being accurate as finger-pricking meters16. Here we present a new sensor based on magnetic resonance spectroscopy (MRS) which is specific to blood glucose (via chemical shift) and has the potential to be as accurate as finger-pricking meter17.METHODS
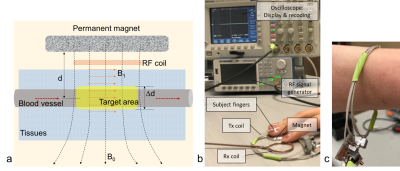
We designed and built a wearable MRS system at 0.1 T (Fig. 1). The B0 field was calculated using the Bio-Savart law in MATLAB (R2018a). The B1+/- field was full-wave simulated using a software (CST microwave studio, Darmstadt, Germany). A signal generator (BK Precision 4065, Yorba Linda, CA) provided RF pulse for the transceive (Tx/Rx) flexible solenoid coil. An oscilloscope (Tektronix TDS3054C, Beaverton, OR) displayed and recorded FID signals which were then demodulated into complex ones and Fourier transformed into MR spectra. Signals from static tissues surrounding blood vessels were saturated by a 1min-long preparation of RF excitation. The asymmetry of water peak caused by small glucose signal was used as glucose index (Eq. 1), which was then transformed into glucose concentration via a linear calibration (Eq. 2). Finger-pricking meter reading was employed as standard reference.Eq. 1: Rgluc = ΔARL / Awater .
Eq. 2: Ygluc = a + b Rgluc .
EXPERIMENTS
The MRS measurement was performed on the tip of a finger (left index) of 30 human subjects (15 Type-2 diabetics and 15 healthy controls; age 45.4±17.0 years in 23-71 years; 10/20 female/male), with an approved IRB and written consents. A custom-developed rectangular RF pulse sequence was used for data acquisition with RF duration=2.5ms and central frequency=3.95MHz, FID readout time =2.5ms, TE/TR=1.25/5ms, averages=17-20. Figure-pricking measurement was performed on subject's finger tips before and after MRS measurement, using a popular meter (OneTouch UltraMini, LifeScan, Switzerland). The two meter readings were averaged. MRS data processing was performed offline in MATLAB (R2019a).RESULTS
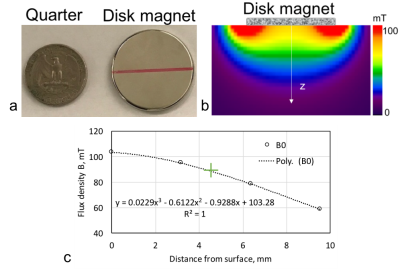
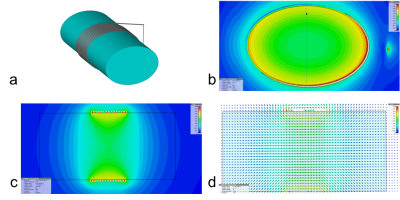
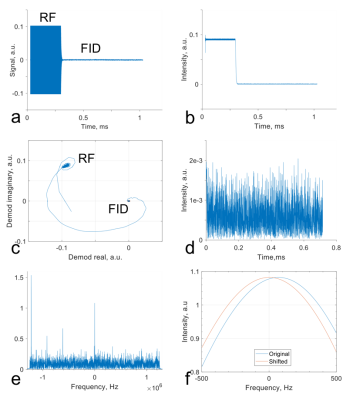
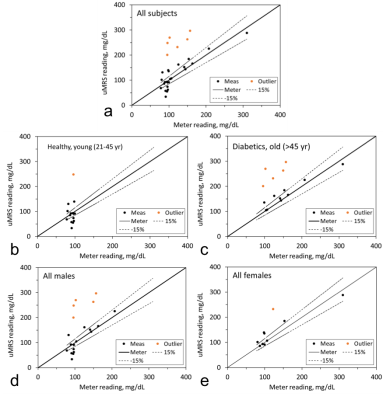
Fig. 2 presents the B0 field of the disk magnet (Fig. 2a) with a 3D simulation in Fig. 2b and a 1D measurement in Fig. 2c. Demonstrated in Fig. 3 are the full wave simulation results of the B1+/- field of a wrist model (εr=80 and σ=0.5 S/m). The signals acquired on a healthy subject were shown in Fig. 4. Fig. 5 shows the measurements of blood glucose on the study subjects. An expected linear relationship between MRS measurement and meter reading was obtained. Statistically, there was no significant difference observed between the male and female subjects, between the healthy and Type-2 diabetics, and between the age groups. However, we also found outliers (1/15 in the healthy subjects and 5/15 in the Type-2 diabetics).DISCUSSION
The B0 field map in Fig. 2 suggests the penetration into the skin at ~6 mm (or 0.8-0.9 T) which is deep enough to reach blood vessels inside tissues. The arrangement of the magnet and coil makes RF field perpendicular to B0 (Fig.3), ensuring production of MR signal. The study results in Fig. 5 show the capability of the wearable MRS system for noninvasive measurement of blood glucose at an accuracy comparable to finger-pricking meter (<15%) and meeting FDA recommendations18,19. We found that the outliers in Fig. 5 were caused by imperfect setup of the measurement such as loose contact between subject’s finger and the coil. This MRS system employed external RF generator and recording subsystems. Other subsystems related to the power supply, signal processing and glucose reading, and safety of users, were not explored in this study.CONCLUSION
This study has shown preliminary data supporting the technical feasibility of proposed wearable MRS system for noninvasive measurement of blood glucose. However, more efforts are needed to minimize the system and build a prototype. Studies on more subjects, especially those with higher (>300 mg/dL) or lower (<100 mg/dL) blood glucose level, are also needed to determine the system’s accuracy and limitations.Acknowledgements
This work was financially supported in part by the Applied Research Support Fund (ARSF) of NYU School of Medicine and the General Research Fund of the Department of Radiology. The authors (Y.Q. and B. Z.) are the inventors of a patent application related to this research.References
1. American Diabetes Association. Standards of medical care in diabetes – 2017. Diabetes Care 2017; 40 (sup 1): s1-s135.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. WHO. Global Report on Diabetes. Geneva, 2016.
4. NCD-Ris C. Worldwide trends in diabetes. Lancet 2016; 387:1513-30.
5. IDF Diabetes Atlas, 7th ed Brussels, Belgium: International Diabetes Federation, 2015 http://www.diabetesatlas.org.
6. Zheng, Y. et al. Global etiology and epidemiology of type 2 diabetes mellitus and its complications Nat. Rev. Endocrinol. 2017.151. doi:10.1038/nrendo.
7. Bestreviews.com. July 2017.
8. https://www.dexcom.com/products. 2018.
9. https://www.freestylelibre.us. 2018.
10. FDA News Release. FDA expands indication for continuous glucose monitoring system, first to replace fingerstick testing for diabetes treatment decisions. Dec 20, 2016.
11. Taylor NP. Apply is testing a noninvasive blood glucose monitor. CNBC; 2017-4-14.
12. Shao J, Lin M, Li Y, Li X, Liu J, et al. In Vivo blood glucose quantification using Raman spectroscopy. PLOS ONE 2012; 7(10): e48127.
13. Otis B, Liao YT, Amirparviz, Yao H. Wireless powered contact lens with glucose sensor system. US Patent Publication No. US20120245444A1. Feb 21, 2012.
14. http://www.gluco-wise.com. 2019.
15. http://www.integrity-app.com/the-glucotrack/. 2019.
16. Smith, John. The pursuit of noninvasive glucose (5th ed): Hunting the deceitful turkey. 2017 https://www.researchgate.net/publication/317267760_The_Pursuit_of_Noninvasive_Glucose_5th_Edition.
17. Qian Y, Zhang B. System and method for blood glucose monitoring using magnetic resonance spectroscopy. U.S. Patent Application No.: 62/618,974. January 2019 Submitted by NYU.
18. FDA. Self-monitoring blood glucose test systems for over-the-counter use: Guidance for industry and Food and Drug Administration staff. Issued on October 11, 2016.
19. ISO 15197:2013. In vitro diagnostic test systems – Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. 2013-05-15.
Figures