1236
Deciphering the Pain-Matrix with Ultrahigh functional MRI: En Route to Objective Biomarkers for Pain1Berlin Ultrahigh Field Facility (B.U.F.F.), Max-Delbrück-Center for Molecular Medicine, Berlin, Germany, 2Experimental and Clinical Research Center, a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
Despite millions of people suffering from chronic pain, clinicians still rely on self-reports for the assessment of pain. In attempts to identify objective biomarkers, fMRI studies have revealed an assembly of regions that consistently activates in response to noxious stimuli, but also to non-noxious equally salient stimuli. Since most studies have been performed at 3T or lower, higher field strengths might be able to differentiate pain from saliency. As a first step, we aimed to identify the pain-matrix at ultrahigh fields using an ON/OFF heat stimulation paradigm and show here that the regions associated with the pain-matrix could be identified.
Introduction
Chronic pain affects 35% of the adult population in the developed world1, which burdens society with escalating costs of medical care, loss of wages, and loss of productivity2,3. The invisible nature and subjectivity of pain makes that we still rely on self-reports for the assessment of pain affecting treatment strategies, payment by health insurance companies1. Consequently, research to address whether and to what extent a patient is in pain or not is needed for a variety of reasons1. While functional MRI (fMRI) has been extensively employed in the hunt for the neurosignature of pain, many regions collectively known as the “pain-matrix” are not pain-specific4-6, as the same network activates in response to non-painful, but equally salient stimuli7-9 and is preserved in pain-free patients10. Limiting factors could be the magnetic field-strength and limited BOLD sensitivity used in previous studies, where ultrahigh field MR could aid in differentiating pain from saliency due to its higher spatial fidelity and enhanced BOLD sensitivity. Recognizing this opportunity, we aimed to identify the pain-matrix at ultrahigh MR fields en route to objective biomarkers for pain.Methods
Experimental setup: Thermal stimulation of 51°C was applied to the left arm of five subjects in 5 blocks of 20 pulses (1 pulse per repetition time [TR]) engaging both the fast A-delta (Aδ-) and slow C- fibers11 using the contact heat-evoked potential stimulator (CHEPS; Medoc Ltd, Ramat-Yoshei, Israel). MR Imaging: High-resolution T2*-weighted fMRI (GE-EPI, TR/TE/FA = 2200ms/28.2ms/66°, FOV/matrix = 240x240mm/160x160, slices = 80, slice thickness = 1.5mm, resolution = 1.5mm isotropic, 220 volumes) was acquired on a MAGNETOM 7T scanner (Siemens Healthcare, Erlangen, Germany) with a single-channel-transmit/32-channel receive head coil (Nova Medical, Wilmington, MA, USA). Data analysis: fMRI data were corrected for motion (SPM; realignment to first volume of the run) and distortion (using the gradient-echo fieldmap approach12-14), smoothed with a 5mm kernel size, registered to the MNI152 template using ANTs, and statistically analyzed using FSL FEAT. The hemodynamic response functions were modeled for the entire stimulus train: 44 sec block, double gamma convolution + temporal derivative with cluster significance thresholds of p<0.05 determined by z-score > 3.1.Results
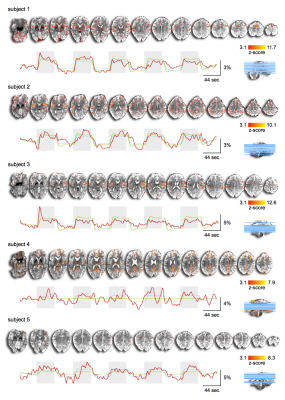
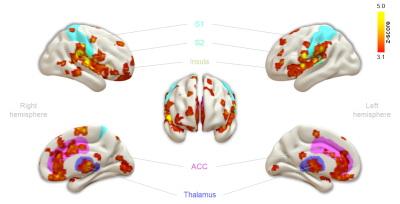
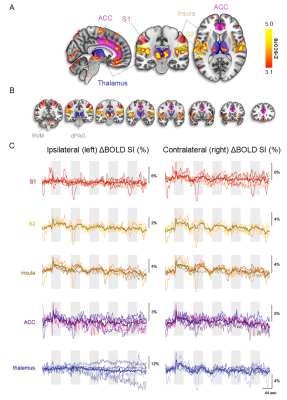
First, we examined the single-subject maps for overall quality and subject-specific activation in response to nociception (Fig. 1; top panel for each subject). All but the last subject showed good agreement in significant BOLD patterns that co-localized with key regions of the pain-matrix. For most subjects, the variance in signal intensity over time was well explained by the statistical model applied (Fig. 1; bottom panel for each subject). After quality assurance of single-subject statistical maps, the individual maps were fed into the group analysis (Fig. 2). This revealed the classical pain-matrix including the somatosensory, anterior cingulate, and insular regions, thalamic (mediodorsal and ventral) and brainstem nuclei (rostroventral medulla [RVM] and periaqueductal grey [PAG]), and the cerebellum (Fig. 2 and 3AB). Significant activity changes were observed in regions of the primary somatosensory cortex contralateral to the stimulation site and remarkable temporal synchronization was observed in the regions associated with the pain-matrix (Fig. 3C), in addition to two other observations: (1) The BOLD response extended into to the rest periods pointing to prolonged nociceptive processing and, (2) The overall magnitude of the signal decreased over time.Discussion
We observed contralateral S1 activation in the limb region while activation in the S2 was bilateral, a feature often observed in human somatosensation due to the bilateral receptive fields of S2-neurons15-17. Additionally, we found widespread thalamic activation including the ventral nuclei that project to the insula18 and mediodorsal nuclei that project to the ACC19, which extensively project to the PAG20-23 and RVM24, together forming the top-down pain control pathway25. Extraction of the time series in clusters of the pain-matrix revealed persistence of the BOLD signal into the rest period, suggesting that canonical modeling of pain based on the simplified ON/OFF stimulation paradigm might paint an incomplete picture of nociceptive processing and that the second component of pain mediated by slow C-fibers should be taken into account26. Additionally, the overall signal magnitude decreased over time in all regions except the rACC, which remains active in most subjects. This region is implicated in antinociceptive processing modulating general habituation in response to repetitive stimulation27-29, presumably modulating the signal magnitude in the other regions over time.Acknowledgements
No acknowledgement found.References
1. Davis KD, Flor H, Greely HT, et al. (2017): Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol. 13:624-638.
2. Von Korff M, Scher AI, Helmick C, et al. (2016): United States National Pain Strategy for Population Research: Concepts, Definitions, and Pilot Data. J Pain. 17:1068-1080.
3. Treede RD, Rief W, Barke A, et al. (2015): A classification of chronic pain for ICD-11. Pain. 156:1003-1007.
4. Hu L, Iannetti GD (2016): Painful Issues in Pain Prediction. Trends Neurosci. 39:212-220.
5. Mouraux A, Iannetti GD (2018): The search for pain biomarkers in the human brain. Brain. 141:3290-3307.
6. Su Q, Qin W, Yang QQ, et al. (2019): Brain regions preferentially responding to transient and iso-intense painful or tactile stimuli. NeuroImage. 192:52-65.
7. Legrain V, Iannetti GD, Plaghki L, et al. (2011): The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 93:111-124.
8. Liang M, Su Q, Mouraux A, et al. (2019): Spatial Patterns of Brain Activity Preferentially Reflecting Transient Pain and Stimulus Intensity. Cereb Cortex. 29:2211-2227.
9. Mouraux A, Diukova A, Lee MC, et al. (2011): A multisensory investigation of the functional significance of the "pain matrix". NeuroImage. 54:2237-2249.
10. Salomons TV, Iannetti GD, Liang M, et al. (2016): The "Pain Matrix" in Pain-Free Individuals. JAMA Neurol. 73:755-756.
11. Craig AD (2013): Chapter 9 - Cooling, pain, and other feelings from the body in relation to the autonomic nervous system. In: Buijs RM, Swaab DF, editors. Handbook of Clinical Neurology: Elsevier, pp 103-109.
12. Cusack R, Brett M, Osswald K (2003): An evaluation of the use of magnetic field maps to undistort echo-planar images. NeuroImage. 18:127-142.
13. Jezzard P, Balaban RS (1995): Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 34:65-73.
14. Jezzard P, Clare S (1999): Sources of distortion in functional MRI data. Hum Brain Mapp. 8:80-85.
15. Khoshnejad M, Piché M, Saleh S, et al. (2014): Serial processing in primary and secondary somatosensory cortex: a DCM analysis of human fMRI data in response to innocuous and noxious electrical stimulation. Neuroscience Letters. 577:83-88.
16. Lamp G, Goodin P, Palmer S, et al. (2019): Activation of Bilateral Secondary Somatosensory Cortex With Right Hand Touch Stimulation: A Meta-Analysis of Functional Neuroimaging Studies. Frontiers in neurology. 9:1129-1129.
17. Treede R-D, Baumgärtner U, Lenz FA (2007): Nociceptive Processing in the Secondary Somatosensory Cortex. In: Schmidt RF, Willis WD, editors. Encyclopedia of Pain. Berlin, Heidelberg: Springer Berlin Heidelberg, pp 1376-1379.
18. Nieuwenhuys R (2012): The insular cortex: a review. Prog Brain Res. 195:123-163.
19. Yang J-W, Shih H-C, Shyu B-C (2006): Intracortical Circuits in Rat Anterior Cingulate Cortex Are Activated by Nociceptive Inputs Mediated by Medial Thalamus. Journal of Neurophysiology. 96:3409-3422.
20. Domesick VB (1969): Projections from the cingulate cortex in the rat. Brain Research. 12:296-320.
21. Hardy SGP, Leichnetz GR (1981): Frontal cortical projections to the periaqueductal gray in the rat: A retrograde and orthograde horseradish peroxidase study. Neuroscience Letters. 23:13-17.
22. Mantyh PW (1982): Forebrain projections to the periaqueductral gray in the monkey, with observations in the cat and rat. Journal of Comparative Neurology. 206:146-158.
23. Morrell JI, Greenberger LM, Pfaff DW (1981): Hypothalamic, other diencephalic, and telencephalic neurons that project to the dorsal midbrain. Journal of Comparative Neurology. 201:589-620.
24. Millan MJ (2002): Descending control of pain. Progress in neurobiology. 66:355-474.
25. Donaldson LF, Lumb BM (2017): Top-down control of pain. The Journal of physiology. 595:4139-4140.
26. Upadhyay J, Pendse G, Anderson J, et al. (2010): Improved characterization of BOLD responses for evoked sensory stimuli. NeuroImage. 49:2275-2286.
27. Bingel U, Schoell E, Herken W, et al. (2007): Habituation to painful stimulation involves the antinociceptive system. Pain. 131:21-30.
28. Bingel U, Herken W, Teutsch S, et al. (2008): Habituation to painful stimulation involves the antinociceptive system--a 1-year follow-up of 10 participants. Pain. 140:393-394.
29. Milne RJ, Kay NE, Irwin RJ (1991): Habituation to repeated painful and non-painful cutaneous stimuli: a quantitative psychophysical study. Exp Brain Res. 87:438-444.
Figures