1233
On the feasibility of using single-shot perfusion labeling (SSPL) at 7 Tesla for laminar fMRI1Advanced MRI section, LFMI, NINDS, National Institutes of Health, Bethesda, MD, United States
Synopsis
Blood oxygen-level dependent (BOLD) functional MRI (fMRI) based on gradient-echo EPI is the most commonly used fMRI method due to its high sensitivity and robustness. However, large vein contribution negatively affects spatial localization of BOLD activation, of crucial importance for laminar and other high-resolution fMRI applications. Perfusion and blood volume-based methods have been shown to increase spatial accuracy of activation maps. Here we demonstrate feasibility of single-shot perfusion labeling (SSPL) fMRI at up to 1 mm3 resolution, a reference-less perfusion fMRI method twice as efficient as FAIR in which background signal is suppressed, improving temporal stability.
Introduction
Blood oxygen-level dependent (BOLD) functional MRI (fMRI) based on gradient-echo echo-planar imaging (EPI) is the most commonly used fMRI method due to its high sensitivity and robustness. However, its fidelity in reflecting the blood flow response to neuronal activity is compromised by the generally dominant contribution of large veins that drain the activated area. This limits the spatial accuracy of typical fMRI experiments to a few millimeters. While special stimulation tasks and processing approaches may ameliorate this problem somewhat, alternative fMRI contrasts less sensitive to venous drainage may be desirable for high resolution studies. For example, both perfusion and blood volume (BV) based fMRI methods have been demonstrated to improve spatial accuracy.1,2,3 These methods suffer from reduced sensitivity however. Here we investigated the utility of Single Shot Perfusion Labeling (SSPL)4 to perform high resolution fMRI experiments, with as ultimate aim laminar fMRI5. SSPL has been demonstrated to improve sensitivity over conventional pulsed labeling based fMRI experiments about 2-fold by using two inversion pulses to suppress stationary tissue signal and eliminate the need for a reference scan. It may also have advantages over BV based methods as heuristically according to Grubb's law,6 perfusion changes substantially exceed BV changes.Materials and Methods
Experiments were performed on a Siemens Magnetom 7T whole-body scanner with 32-channel Nova Medical receive coil using an in-house developed pulse sequence that allowed conventional BOLD-EPI as well as SSPL-EPI. To optimize parameters for 7 T, data were acquired using 23 mm3 (8 μl) voxels, rate-2 SENSE, 3 s TR and 17.5 ms TE. A multi-slice implementation was investigated in which several imaging slices were acquired around the nominal second inversion time (TI2). Here 5-slice SSPL was acquired; BOLD-EPI with similar resolution and echo time allowed the acquisition of 45 slices. A FAIR-like option was added to the SSPL sequence in which inversion was non-selective every other TR to allow contrast interpretation and perfusion quantification. Two slice-selective hyperbolic secant pulses (10240 μs duration; 800 Hz amplitude; 30/120 mm thickness) were used for the inversions. Functional MRI used a 7.5 Hz full-field checkerboard task with 30 s off/30 s on blocks, ~5.5 min scan duration (110 repetitions). A limited number of high-resolution scans with 13 mm3 (1 μl) resolution were also acquired (n=3, rate-3 SENSE; 38 ms TE; 5 slices; 110 or 210 repetitions; BOLD: 33 slices).Results and Discussion
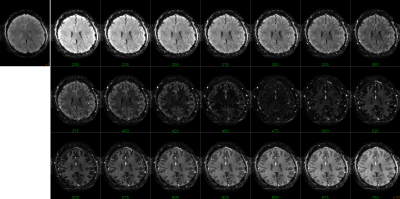
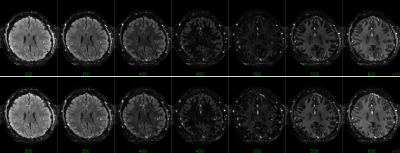
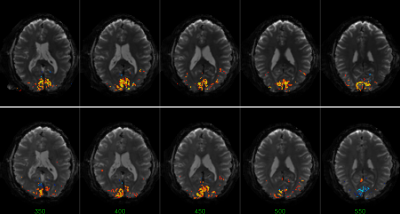
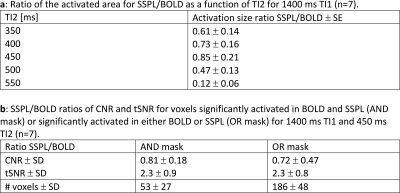
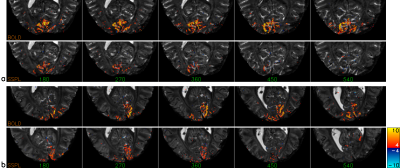
The combination of TI1 and TI2 was optimized for simultaneous suppression of gray and white matter. TI1 was fixed at 1400 ms, optimal background signal suppression was then obtained around 450-475 ms TI2 (Figure 1). Slice excitation and EPI readout took almost 50 ms, therefore most 5-slice SSPL experiments were performed using 350, 400, 450, 500 and 550 ms TI2. Effects of additional RF pulses to excite neighboring slices were not observed when comparing the 5-slice approach to comparable conventional 1-slice SSPL (Figure 2). This was supported by FAIR-like data from similar 5-slice experiments for various TI2 ranges (data not shown). Robust activation was found for TI2 ≤450 ms, longer TI2s showed substantially reduced activation (Figure 3 and Table 1). Note that the sign of the activation was inverted for longer TI2 values since the sign of the background signal had flipped. Very low background signal (~450 ms TI2) was found to hamper analysis, as either real, task-induced signal changes, or noise, could change signal sign, an effect lost in the analysis of magnitude data that can only be partially addressed by analysis based on complex signal (under development). Overall the level of activation observed in SSPL was reduced compared to BOLD, both when comparing activation size and contrast-to-noise ratio (CNR) (Table 1). Note however that the reduced activation size could in part reflect improved localization of the activation. SSPL's reduced sensitivity to physiological fluctuations is evident from the ~two-fold higher temporal stability (tSNR) at 8 μl resolution (Table 1). Preliminary data acquired at the higher 1 μl resolution reflect these findings (e.g. see Figure 5), showing that activation can be measured using SSPL fMRI at 1 μl resolution, albeit with BOLD showing more robust activation. Additional high-resolution data will be required to address the question of improved spatial localization of SSPL when compared to BOLD.Conclusion
The feasibility of high-resolution SSPL for perfusion-based fMRI at high field, at up to 1 μl resolution, was demonstrated. Whereas BOLD fMRI at the same resolution provides both higher sensitivity and coverage, spatial specificity concerns make non-BOLD approaches of interest for high-fidelity laminar fMRI.Acknowledgements
This work was supported by the Intramural Research Program of NINDS, National Institutes of Health.References
1. Duong TQ, Kim DS, Uǧurbil K, Kim SG. Localized cerebral blood flow response at submillimeter columnar resolution.Proc Natl Acad Sci U S A 2001;98:10904-10909
2. Lu H, Golay X, Pekar JJ, van Zijl PC. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med 2003;50:263-274
3. Huber L, Uludaǧ K, Möller HE. Non-BOLD contrast for laminar fMRI in humans: CBF, CBV, and CMRO2. Neuroimage 2019;197:742-760
4. Duyn JH, Tan CX, van Gelderen P, Yongbi MN. High-sensitivity single-shot perfusion-weighted fMRI. Magn Reson Med 2001;46:88-94
5. Polimeni JR, Fischl B, Greve DN, Wald LL. Laminar analysis of 7 T BOLD using an imposed spatial activation pattern in human V1. Neuroimage 2010;52:1334-1346
6. Grubb RL, Phelps ME, Eichling JO. The effects of vascular changes in PaCO2 on cerebral blood volume, blood flow and vascular mean transit time. Stroke 1974;5:630–639
Figures