1232
In-vivo laminar CBF fMRI using high-resolution pseudo-continuous arterial spin labeling at 7T1Mark & Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Department of Neurology, University of Southern California, Los Angeles, CA, United States, 3State Key Laboratory of Brain and Cognitive Science, Beijing MRI Center for Brain Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
Synopsis
In-vivo laminar CBF fMRI was performed by high resolution (0.5×0.5×1.5 mm3) inner-volume GRASE with optimized pCASL labeling at 7T. Activation of finger-tapping task (5 blocks, TA=10 min) was reliably detected in all 4 subjects. Both rest/FT CBF peaks in the middle layers, which corresponds to highest capillary density in cortex layer IV. FT evoked CBF increase shows one peak in middle layer, and a second shoulder in deep layer. The capability to provide quantitative CBF measurements at both baseline and task activation with high specificity to neuronal activities is a unique strength of ASL fMRI compared to other fMRI techniques.
Introduction
Cerebral blood flow (CBF) fMRI measured by arterial spin labeling (ASL) is less affected by susceptibility effect and has higher spatial specificity as compared to BOLD. However, low SNR limits the sensitivity of CBF fMRI, and it is challenging to detect CBF response to neuronal activation in layers. Ultra-high field ASL has benefitted from significant higher SNR due to longer longitudinal relaxation time of arterial blood1 and super-linearly increased SNR with field strength.2 We demonstrated that pseudo-continuous arterial spin labeling (pCASL) with 3D inner-volume gradient and spin echo (GRASE) readout has the capability of revealing layer dependent CBF at 7T.3 In this study, we extended the previous work to acquire laminar CBF fMRI in motor cortex with optimized pCASL labeling scheme.Methods
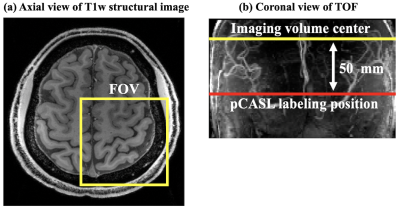
Four healthy subjects (2M, age=26.0±4.8 yrs) underwent MRI scans on a 7T Siemens Terra scanner with a NOVA 1Tx/32Rx head coil. GRASE readout with inner-volume excitation was used to acquire a small imaging volume covering left motor cortex. pCASL labeling plane was placed above the circle of Willis (CoV) and 50 mm below imaging volume center, as shown in Figure 1.Imaging parameters were: FOV = 100mm, matrix size = 96 × 96, 8 slices (20% oversampling), resolution = 0.5×0.5×1.5 mm3 (in-plane interpolation), 2 segments along phase direction, bandwidth = 1580 Hz/pixel, TE = 33.2 ms, labeling duration = 1280 ms, PLD = 800 ms, TR = 2500 ms. CBF fMRI task consisted of five blocks of 60 secs bilateral sequential finger-tapping (FT) interspaced by 60 secs of rest. Total scan time = 10 mins 15 secs.
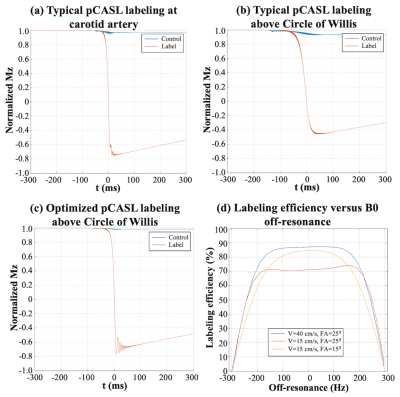
Labeling efficiency was derived by Bloch equation simulations. Flip angle of pCASL pulses and average gradient strength were optimized for slower flow velocity in the middle cerebral artery. Optimal parameter was chosen for achievable highest labeling efficiency with the constraint of SAR limit. SAR was monitored by FDA approved vendor software and was within the first level (3.2W/kg on head).
Rigid head motion correction was performed off-line using SPM. Rest/FT CBF maps were calculated according to ASL white paper,4 and average CBF values were measured in 3 manually segmented gray matter layers based on co-registered T1w MP-RAGE images (0.7mm isotropic) using ITK-SNAP.5 A general linear model (GLM) was used to detect voxels of statistical significance (FT > rest, p < 0.05, uncorrected). ROIs were defined as significant vertices from the surface. Gray matter voxels corresponding to the surface ROI were interpolated with nearest neighbor method into 12 equi-volume bins. Within-layer smoothing (1mm FWHM gaussian window) was performed for illustration purpose in Figure 5. AFNI/SUMA, FreeSurfer and custom Python/Matlab codes were used for layer-specific data analysis.
Results and discussion
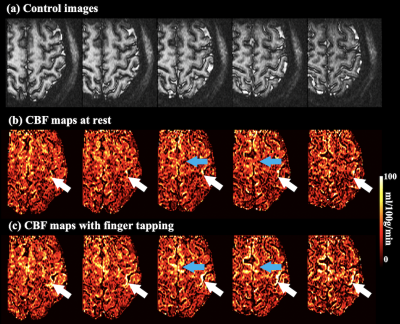
Labeling efficiency = 87% with typical pCASL parameter (FA=250, Gmean=0.6 mT/m) labeling at carotid artery (V = 40 cm/s) (figure 2.(a)), however, labeling efficiency reduced to 71% when labeling above the CoV (V = 15 cm/s) (figure 2.(b)). With optimized parameter (FA=150, Gmean=1 mT/m), 85% labeling efficiency (figure 2.(c)) can be achieved with 64% lower SAR and good off-resonance resistance (figure 2.(d)).Figure 3 (a) shows a reference inner-volume GRASE image. Rest/FT CBF maps were shown in figure 3 (b) and (c), respectively. Strong CBF increase evoked by the FT task can be observed along the primary motor cortex (M1) and in supplementary motor area (SMA), as indicated by white and blue arrows.
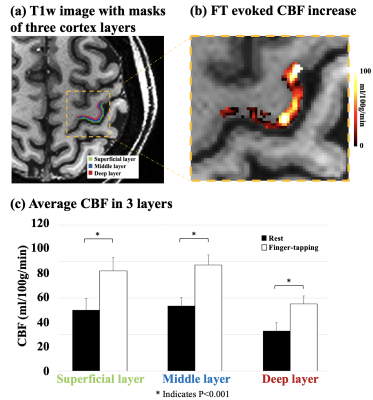
Figure 4 (a) shows the co-registered MP-RAGE image with manually segmented 3 cortical layers in M1. Figure 4 (b) shows FT evoked CBF increase in an enlarged ROI. Average CBF in superficial, middle and deep layer are 33.3±6.4, 35.7±4.7 and 22.0±4.6 ml/100g/min at rest, and 54.9±7.4, 58.1±5.5 and 36.7±4.3 ml/100g/min with FT (figure 4 (c)). FT task evoked absolute CBF increase were 21.6, 22.4 and 14.8 ml/100g/min, and relative CBF increase were 64.6%, 62.8% and 67.2% in superficial, middle and deep layer, respectively.
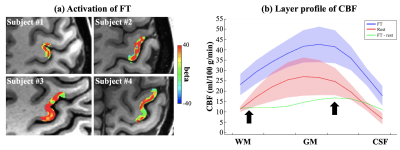
Activation of FT was reliably detected with GLM in all 4 subjects, as shown in figure 5 (a), which suggests that the proposed technique provides sufficient SNR for laminar CBF fMRI studies. Figure 5 (b) shows the layer profile averaged from 4 subjects. Both rest/FT CBF peaks in the middle layers, which corresponds to highest capillary density in cortex layer IV.6 FT evoked CBF increase shows one peak in middle layer, and a second shoulder in deep layer, which follows the ‘two-peak’ activation pattern as reported in a previous VASO fMRI study at 7T.7 This is in contrast to depth-dependent BOLD fMRI that shows higher signal changes towards the cortical surface due to pial veins.
Conclusion
In-vivo laminar CBF fMRI was performed by high resolution inner-volume GRASE with optimized pCASL labeling at 7T. The capability to provide quantitative CBF measurements at both baseline and task activation with high specificity to neuronal activities is a unique strength of ASL fMRI compared to other fMRI techniques.Acknowledgements
This work was supported by National Institute of Health (NIH) grant UH3-NS100614 and R01-EB028297.References
[1] Zuo Z, Wang R, Zhuo Y, et al. Turbo-FLASH based arterial spin labeled perfusion MRI at 7 T[J]. PloS one, 2013, 8(6): e66612.
[2] Pohmann R, Speck O, Scheffler K. Signal‐to‐noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays[J]. Magnetic resonance in medicine, 2016, 75(2): 801-809.
[3] Shao X, Wang K, Wang DJJ, 7T high-resolution arterial spin labeling reveals layer dependent cerebral blood flow. ISMRM, 2019.
[4] Alsop D C, Detre J A, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia[J]. Magnetic resonance in medicine, 2015, 73(1): 102-116.
[5] Paul A. Yushkevich, Joseph Piven, Heather Cody Hazlett, Rachel Gimpel Smith, Sean Ho, James C. Gee, and Guido Gerig. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006 Jul 1;31(3):1116-28.
[6] Lauwers F, Cassot F, Lauwers-Cances V, et al. Morphometry of the human cerebral cortex microcirculation: general characteristics and space-related profiles[J]. Neuroimage, 2008, 39(3): 936-948.
[7] Huber L, Handwerker D A, Jangraw D C, et al. High-resolution CBV-fMRI allows mapping of laminar activity and connectivity of cortical input and output in human M1[J]. Neuron, 2017, 96(6): 1253-1263. e7.
Figures