1231
Mapping directional functional connectivity across brain-wide networks with layer-specific CBV-fMRI1MBIC, Uni Maastricht, Faculty of Psychology and Neuroscience, Maastricht, Netherlands, 2SFIM, NIMH, Bethesda, MD, United States
Synopsis
With recent advances in ultra-high-field MRI hardware and sequence mechanisms, it has become possible to measure CBV-weighted fMRI signal across cortical layers. While initial proof-of-principle layer-fMRI studies in primary brain areas with conventional fMRI task designs are promising, layer-fMRI has not yet realized its full potential to map layer-dependent functional connectivity across large-scale brain networks. In this study, we investigate the applicability of CBV-weighted layer-fMRI to assess functional connectivity during resting-state and naturalistic tasks. We can map common resting-state networks and characterize their internal layer-dependent signatures with respect to directionality and cortical hierarchy.
Purpose
Methodological advances in functional Magnetic Resonance Imaging (fMRI) in recent years allow researchers to approach the mesoscopic spatial regime of cortical layers and cortical columns [Huber 2018]. This revolutionizes the ability to tackle cortical information processing within brain systems [Polimeni 2010, Koopmans 2011, Muckli 2015, Kok 2016, de Martino 2015, Huber 2017]. However, due to sensitivity constraints and venous signal leakage effects, most layer fMRI studies have been confined to primary cortices in combination with basic fMRI task block-designs. To become an established and user-friendly method for applications in cognitive neuroscience [Finn 2019], layer-fMRI acquisition and analysis methods must be extended to more sophisticated task designs and higher-order cognitive areas. In this study, we investigate the neuroscientific applicability of layer-fMRI to map the functional connectome across large-scale, brain-wide functional networks with layer specificity.- We discuss the sensitivity requirements for layer-fMRI with naturalistic tasks of free movie watching compared to resting-state (Fig. 1).
- We assess the necessity of locally specific non-BOLD contrasts in the context of tasks with CNR-starved effect sizes (Fig. 2-3).
- We characterize the capabilities and challenges of layer-fMRI to generate participant-specific directional connectomes with resting-state and movie watching data. Here we focus on the test cases of already established functional networks including: default mode network, visual networks, fronto-parietal networks, and many more (Fig. 3-4).
- We discuss potential future analysis strategies to assess features of cortical hierarchy (Fig. 5).
Methods
Multiple 14-min acquisitions were conducted during resting-state and/or movie watching in N=11 participants Sequence development was done in Maastricht and approved by Maastricht ethical review board: ERCPN:180_03_06_2017. Nine out of the eleven participants were acquired under the NIH Combined Neuroscience Institutional Review Board-approved protocol (93-M-0170, ClinicalTrials.gov identifier: NCT00001360). Functional data of GE-BOLD and cerebral blood volume (CBV) were concomitantly acquired with SS-SI-VASO. A slab-selective (28 slices) and a whole brain VASO sequence (104 slices) were used (for more details on the sequence approach see Huber et al., submitted to ISMRM https://layerfmri.page.link/WholeBrain_ISMRM20). fMRI readout parameters were: in-plane resolution 0.79mm, slice-thickness: 0.8mm, TE=25ms, in-plane PF=6/8 with POCS8, FLASH-GRAPPA=3, TR=8.4s, 3D-EPI readout [Poser 2010], ‘classic’ 7T Magnetom (Siemens Healthineers), 32-ch NOVA coil, SC72 body gradient.Results and Discussion
- We find that the ability to perform signal averaging across multiple movie-watching runs is highly beneficial in layer-fMRI to extract more neurally driven ICA components. The improved sensitivity in movie watching tasks compared to ‘resting-state’ tasks allows for the extraction of unique brain networks (Fig. 1).
- When CBV-sensitive VASO contrasts are acquired in tandem with conventional GE-BOLD, the high-CNR of BOLD can be combined with the high localization specificity of VASO. When the ROI selection and seed time course extraction is guided by the high-CNR of BOLD signals, VASO fMRI can provide data with improved neuroscientific interpretability across cortical depths without venous biases (Fig. 2).
- Despite the limited SNR and less efficient temporal sampling in sub-millimeter fMRI, it is possible to capture large brain networks with layer-specific localization accuracy (Fig. 3).
- We find that it is possible to pinpoint feed-forward and feedback connections between V1 and V5, as well as between BA3B and BA1 as off-diagonal connectivity elements (Fig. 4). However, to account for shared sources of signal fluctuations across cortical depths (e.g. physiological noise), it is vital to normalize any measure of functional connectivity with a reference seed region.
- With iterative, seed-based clustering analysis algorithms (Fig. 5), it is possible to map the functional hierarchy in the visual system of the brain.
Conclusion
Layer-dependent fMRI is limited by SNR and venous signal leakage, making it challenging to map the functional connectome in humans. However, with the application of naturalistic tasks (free movie watching), and with the application of locally specific CBV-weighted fMRI, we found that it is possible to measure functional connectivity across the entire brain without biases of venous signal leakage, yielding layer-dependent localization specificity.The ability to reliably measure the whole brain layer-dependent connectome without venous biases constitutes a paradigm shift in human neuroscience.
It can be a valuable tool:
- to obtain information about directional information flow across the entire brain
- to reveal a causal relationship in functional large scale networks
- to map out the functional hierarchy along multi-area processing streams.
Future efforts will focus on measuring the whole brain layer-dependent connectome matrices across more participants and provide them as an open dataset.
Acknowledgements
- Laurentius Huber is funded form the NWO VENI project 016.Veni.198.032.
- Benedikt Poser received partial funding from 16.Vidi.178.052.
- Benedikt Poser and Sriranga Kashyap received funding from R01 MH111444/MH/NIMH NIH.
- Yuhui Chai, Peter Bandettini and Sean Marrett are funded from the Intramural Research Program of the National Institute of Mental Health (ZIAMH002783).
- Rainer Goebel received funding from the European FET Flagship project ‘Human Brain Project’ (FP7-ICT-2013-FET-F/604102 Grant Agreements, No. 7202070 (SGA1) and No. 785907 (SGA2)).
References
- De Martino et al. 2015. “Frequency Preference and Attention Effects across Cortical Depths in the Human Primary Auditory Cortex.” PNAS 112(52): 16036–41.
- Huber et al. (2018). In 48th Society for Neuroscience, p. 675.03.
- Finn et al. (2019). Nature Neuro.Huber et al. (2018). Neuroimage in press.Huber et al. (2017). Neuron.
- Huber et al. (2017). Neuroimage, doi: 10.1016/j.neuroimage.2017.07
- Muckli et al. (2015). Contextual Feedback to Superficial Layers of V1 Report Contextual Feedback to Superficial Layers of V1. Curr. Biol. 25, 2690–2695.
- Kok et al. (2016). “Selective Activation of the Deep Layers of the Human Primary Visual Cortex by Top-down Feedback.” Current Biology 26(3): 371–76. http://dx.doi.org/10.1016/j.cub.2015.12.038.
- Koopmans et al. (2010). “Layer-Specific BOLD Activation in Human V1.” Human Brain Mapping 31(9): 1297–1304.
- Poser et al. (2010). “Three Dimensional Echo-Planar Imaging at 7 Tesla.” NeuroImage 51(1): 261–66. http://dx.doi.org/10.1016/j.neuroimage.2010.01.108 (July 15, 2012).
- Polimeni et al. (2010). ISMRM (Vol. 18, p. 353).
- Sharoh et al. (2019). “Laminar Specific FMRI Reveals Directed Interactions in Distributed Networks During Language Processing.” PNAS: 1907858116. https://doi.org/10.1073/pnas.1907858116.
- Smith et al. (2009). “Correspondence of the Brain’s Functional Architecture during Activation and Rest.” Proceedings of the National Academy of Sciences 106(31): 13040–45.
Figures
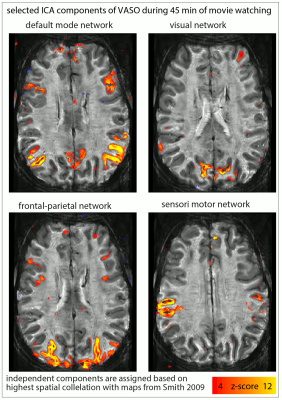
Fig. 1: Usability of resting-state vs. movie watching tasks (45 min each) for ICA decomposition at SNR-starved sub-millimeter voxels (animated gif, please click on it):
The limited tSNR of resting-state layer-fMRI is a challenge when using ICA to detect meaningful components. For movie watching tasks, however, the ability to average across runs allows more efficient extraction of the common networks. The corresponding ICs are selected across tasks based on spatial correlation to reference maps from (Smith 2009).
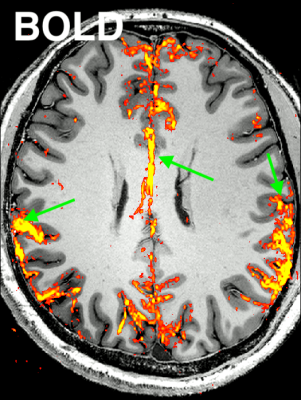
Fig. 2: Representative result of the “Default Mode Network” for CBV-weighted VASO and BOLD (animated gif, please click on it):
Here, an ICA is initially performed on a combined time series (merging BOLD and VASO time series). Next, the signal trace of the DMN component is selected and used as a regressor in a GLM analysis for VASO and BOLD individually to extract beta-value maps. It can be seen that BOLD has multiple false positive voxels at the location of large vessels (green arrow), compared to VASO.
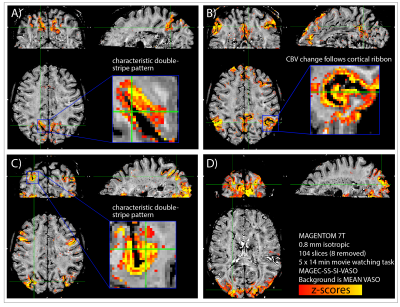
Fig. 3: Results from a representative participant: Whole-brain sub-millimeter data reveal common large-scale functional networks with unique layer-dependent signal distributions.
Here, we used manually selected average signal traces of HCP participants during movie watching as regressors in a GLM analysis to obtain functional maps. The most common and robust networks are the parietal network (A), the default mode network (B), the fronto-parietal network (C) and the visual network (D).
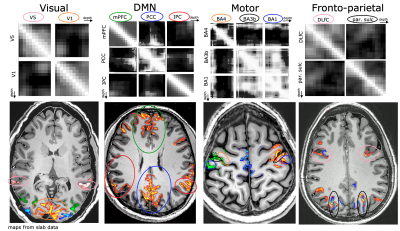
Fig. 4: Representative results of layer-to-layer functional connectivity matrices in selected functional networks.
The top row refers to resting-state correlations between temporal fluctuations in the voxel-averaged VASO signal of layers, across and within brain areas. Note that the off-diagonal elements are specific to individual layers.The bottom row refers to the corresponding activation maps in representative participants during movie watching tasks.
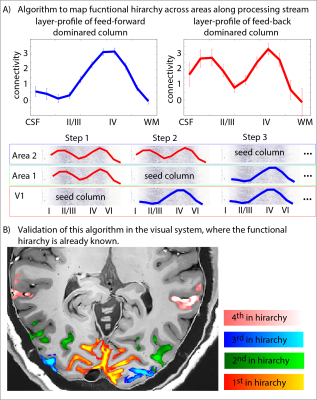
Fig. 5: Functional hierarchy mapping with layer-dependent clustering analysis.
Here we investigate potential data-driven analysis algorithms to map out the functional hierarchy. Without assuming predefined ROIs, we cluster each cortical column in one of two categories (A): feedforward driven (single middle layer peak) vs. feed-back driven (additional superficial peak). Panel (B) shows the applicability of this algorithm in the toy model of the visual system.