1230
Mapping ocular dominance columns in humans using GE-EPI, SE-EPI and SS-SI-VASO at 7 T1Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2International Max Planck Research School on Neuroscience of Communication: Function, Structure, and Plasticity, Leipzig, Germany, 3Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Department of Cognitive Neuroscience, Maastricht Brain Imaging Center, Maastricht, Netherlands, 6Department of Psychology, San Diego State University, San Diego, CA, United States
Synopsis
Functional MRI studies classically rely on the use of GE-EPI sequences. However, the GE-based signal is inherently sensitive to large veins, which impairs its use in high-resolution fMRI application. Other BOLD- and CBV-based approaches like SE-EPI and SS-SI-VASO, respectively, promise a higher specificity at the expense of sensitivity. In the present work, we tested if ocular dominance columns (ODCs) can be detected using GE-EPI, SE-EPI and SS-SI-VASO at 7 T. ODCs could be reliably mapped using all three acquisition methods. Furthermore, we could show for the first time ODCs in humans by exploiting the functional CBV response using SS-SI-VASO.
Introduction
With the advent of functional magnetic resonance imaging at ultra-high magnetic fields (≥ 7 T), mapping of small physiological structures like ocular dominance columns (ODCs) in the primary visual cortex (V1) became possible in vivo. 1,2 However, using GE-EPI, which is the choice in most fMRI applications, the acquired signal is inherently sensitive to large draining veins 3, which reduces its specificity towards the source of neural activation. Other approaches like SE-EPI and especially SS-SI-VASO 4 promise higher specificity towards the microvasculature at the expense of sensitivity by attenuating extravascular signal contributions from larger veins and exploiting a more specific contrast mechanism like cerebral blood volume (CBV), respectively. In this study, we test if we can reliably detect ODCs in humans using GE-EPI, SE-EPI and SS-SI-VASO. To the best of our knowledge, we show for the first time ODCs in humans by measuring the functional CBV response with SS-SI-VASO.Methods
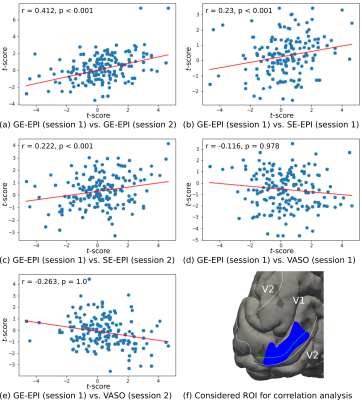
Experiments were performed on a 7 T whole-body MR scanner (Siemens Healthineers, Germany) using a 32 channel phased array head RF coil (Nova Medical Inc, USA). The study was carried out with approval from the local Ethics Committee and written informed consent was obtained. One participant was invited for 7 scanning sessions on different days. While the first session was used for measurements of anatomical reference and retinotopy 5, ODCs were measured in the remaining sessions with each sequence type twice. ODCs were localized by alternate visual stimulation of single eyes using moving sparse random dot stereograms viewed through in-house constructed anaglyph goggles. 6 In each session, 10 functional runs of 270 s length were acquired. For acquisition, a slab was positioned in an oblique-coronal fashion over the posterior end of the occipital lobe. For GE-EPI / SE-EPI protocols, we used the CMRR MB sequence 7,8 without multiband acceleration, TR = 3000 ms, TE = 24 ms / 38 ms, FA = 78 deg / 90 deg. SS-SI-VASO was used with a 3D EPI readout 4, TR = 2500 ms, TE = 25 ms, FA = 26 deg and TI = 650 ms. All protocols had the same nominal isotropic voxel size (0.8 mm)3, field-of-view and effective acceleration with GRAPPA = 3 and partial Fourier 6/8 along the in-plane phase-encoding direction. The number of slices had to be reduced for SE-EPI due to SAR limitations. SPM12 (Functional Imaging Laboratory, University College London, UK) was used for GLM analysis without spatial smoothing (though the applicability of the same inferential statistics for VASO might not be fulfilled). To correct for BOLD modulations in the VASO time series, dynamic division of interleaved acquired nulled and not-nulled volumes was performed. 4 Anatomical data was automatically segmented using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). For profile sampling, reconstructed surfaces were flattened and re-sampled onto a cartesian grid. Then, sampled data from different cortical depths were stacked together to form a 3D array. 9 Since ground truth for ODC detection is missing by relying only on fMRI data, we performed a test-retest analysis in a representative ROI and measured the vertex-wise correlation between sessions, expecting a high reliability within the stimulated visual field of V1 (see fig. 3 for further explanation).Results
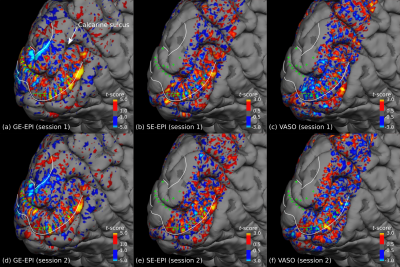
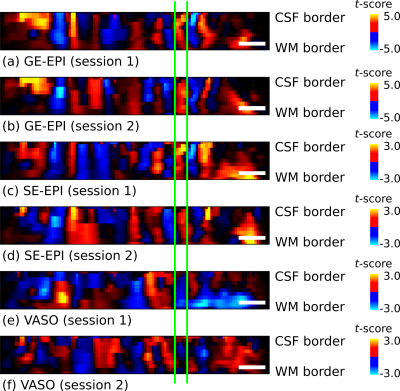
Fig. 1 shows thresholded t-maps of ODCs (left eye > right eye) on the left hemisphere close to the vertical meridians sampled at the central cortical layer and represented on the pial surface. The expected topography with ODCs radiating from the V1 border into the calcarine sulcus 10 can be seen in all sessions. Due to lower sensitivity of SE-EPI and SS-SI-VASO, the threshold was lowered which increases the false positive rate (e.g. outside of V1). However, several ODCs can be clearly seen within V1 and coincide across sessions and sequence types (see green crosses in fig. 1). Note that VASO shows the inverse contrast, since the MR signal of gray matter tissue is reduced after neural activation due to vessel dilation. The profile across cortical depth along the dashed green line is shown in fig. 2. The columnar nature of ODCs can be seen across sessions as well. To further examine the reliability of activation patterns between sessions, a vertex-to-vertex correlation within a defined patch in V1 was analyzed, shown in fig. 3. While the expected trend of the regression line can be revealed in all sessions, only GE-EPI and SE-EPI show a statistically significant correlation.Discussion and conclusion
A robust pattern of ODCs could be revealed across days and sequences in humans using BOLD- and CBV-based fMRI approaches. While ODCs can be observed in each session, a statistical comparison between GE-EPI and SS-SI-VASO showed the expected negative correlation but without statistical significance. This is not unexpected due to the lower sensitivity of VASO and more subjects are needed to get a more conclusive picture. The presented approach allows for in depth comparisons of these different contrasts and acquisition methods in terms of their sensitivity and specificity to neural activation. For example, the inherent cortical depth-dependent vascular blurring can be studies as previously proposed. 9 In conclusion, the study demonstrates for the first time the feasibility of measuring ODCs in humans using GE-EPI, SE-EPI and SS-SI-VASO.Acknowledgements
We thank the University of Minnesota Center for Magnetic Resonance Research for the provision of the multiband EPI sequence software. Furthermore, we thank Dr. Toralf Mildner for insightful discussions. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n° 616905.References
1. Menon RS, Ogawa S, Strupp JP, et al. Ocular dominance in human V1 demonstrated by functional magnetic resonance imaging. J Neurophysiol 1997; 77(5): 2780-87.
2. Yacoub E, Shmuel A, Logothetis N, et al. Robust detection of ocular dominance columns in humans using hahn spin echo BOLD functional MRI at 7 tesla. Neuroimage 2007; 37(4): 1161-77.
3. Polimeni JR, Fischl B, Greve DN, et al. Laminar analysis of 7 T BOLD using an imposed spatial activation pattern in human V1. Neuroimage 2010; 52(4): 1334-46.
4. Huber L, Handwerker DA, Jangraw DC, et al. High-resolution CBV-fMRI allows mapping of laminar activity and connectivity of cortical input and output in human M1. Neuron 2017; 96(6): https://doi.org/10.1016/j.neuron.2017.11.005.
5. Sereno MI, Dale AM, Reppas JB, et al. Borders of multiple visual areas in humans revealed by functional resonance imaging. Science 1995; 268(5121): 889-93.
6. Nasr S, Polimeni JR, and Tootell RBH. Interdigitated color- and disparity-selective columns within human visual cortical areas V2 and V3. J Neurosci 2016; 36(6): 1841-57.
7. Moeller S, Yacoub E, Olman CA, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med 2010; 63(5): 1144-53.
8. Feinberg DA, Moeller S, Smith SM, et al. Multiplexed echo planar imaging for sub-second whole brain fMRI and fast diffusion imaging. PLoS One 2010; 5(12):e15710.
9. Haenelt D, Weiskopf N, Mueller R, et al. Reliable 3D mapping of ocular dominance columns in humans using GE-EPI fMRI at 7 T. Proceedings of the 25th Annual meeting of the Organization for Human Brain Mapping (OHBM) 2019: https://doi.org/10.13140/RG.2.2.21753.21609.
10. LeVay S, Connolly M, Houde J, et al. The complete pattern of ocular dominance stripes in the striate cortex and visual field of the macaque monkey. J Neurosci 1985; 5(2): 486-501.
Figures